
Which of the following pairs form the same osazone?
A) Glucose and Fructose
B) Glucose and Galactose
C) Glucose and Arabinose
D) Lactose and Maltose
Answer
585.9k+ views
Hint: The osazone formation is a characteristic reaction of active groups like carbonyl group as aldehyde and ketone. The osazone is formed by the action of phenylhydrazine on sugar. In excess of reagent, the sugar is converted into diphenyl hydrazone of the sugar. The reagent can convert the carbonyl group to the phenyl hydrazine residue. The general for the aldehyde is as shown below:
\[\text{R}-\text{CHO}\xrightarrow{{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{NHN}{{\text{H}}_{\text{2}}}}\text{ R}-\text{CH}=\text{N}-\text{NH}-{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\]
Complete Step by step answer:
Sugar reacts with the excess of phenyl hydrazine to form phenyl hydrazone. This is called the osazone and the reaction is called the osazone formation reaction.
The aldehyde reacts with the [phenyl hydrazine to form phenyl hydrazine. However, the sugar which has aldehyde or ketone group, on warming up with the excess of phenylhydrazine reagent gives diphenyl hydrazones.
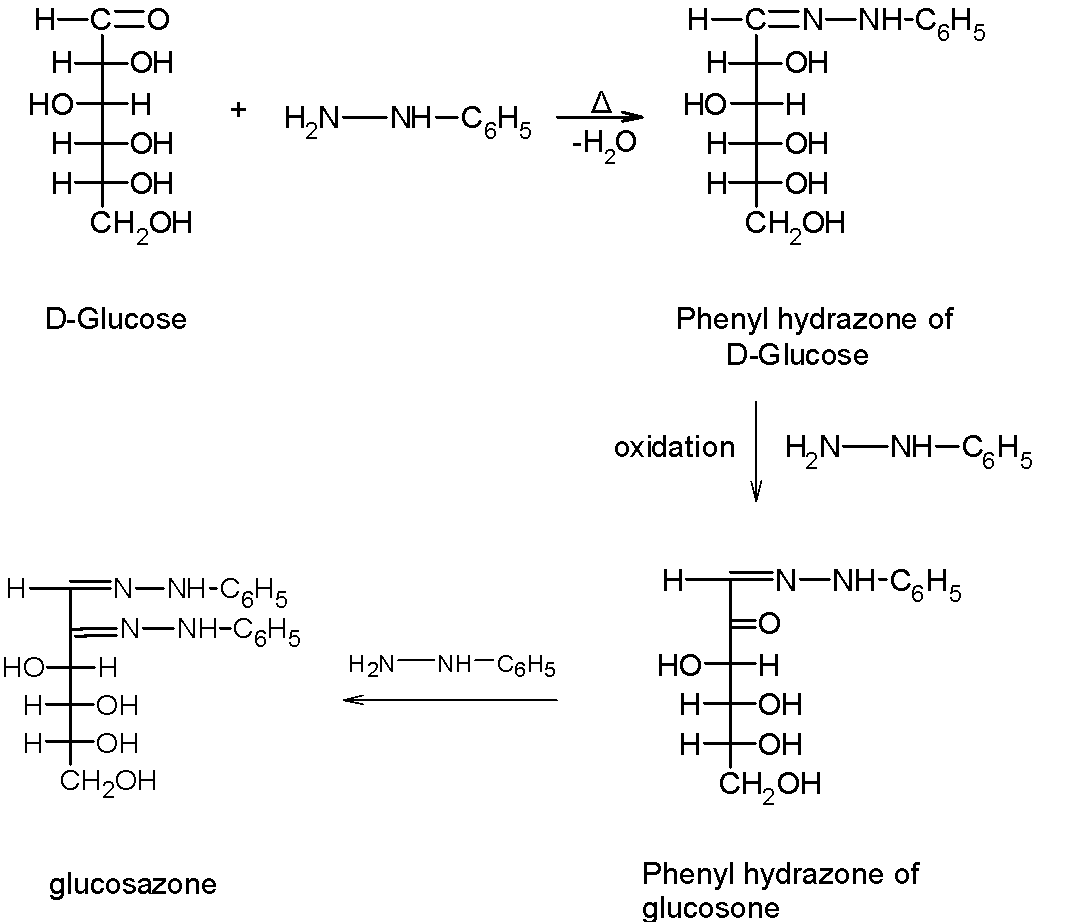
For example, let's consider that glucose reacts with the excess of phenylhydrazine to give the corresponding phenylosazone, which is glucosazone. The reaction takes place in three steps:
Step 1) The first molecule of phenyl hydrazine reacts with the aldehyde $\text{ }(-\text{CHO) }$group of the glucose to give the glucose phenylhydrazine. During this step, the one equivalent of the hydrazine is utilized to generate phenyl hydrazine.
Step 2) In step 2, the second molecule of the phenyl hydrazine oxidizes the reactive $\text{ }\alpha -\text{ }$hydroxyl group to generate the carbonyl group. Since, the aldehyde has already reacted to give hydrazine, the next equivalent of reagent attacks on the hydroxyl group on the adjacent carbon atom, oxidizes it to the carbonyl group.
Step 3) finally, the third molecule of the phenyl hydrazine reacts with the earlier formed carbonyl group and converts into hydrazine. Now, the glucose molecules have two phenyl hydrazone groups thus it is called the diphenyl hydrazine or glucosazone.
The progress of the reaction is as shown below,
Similarly, let's consider an example of fructose. The fructose undergoes the osazone formation as follows:
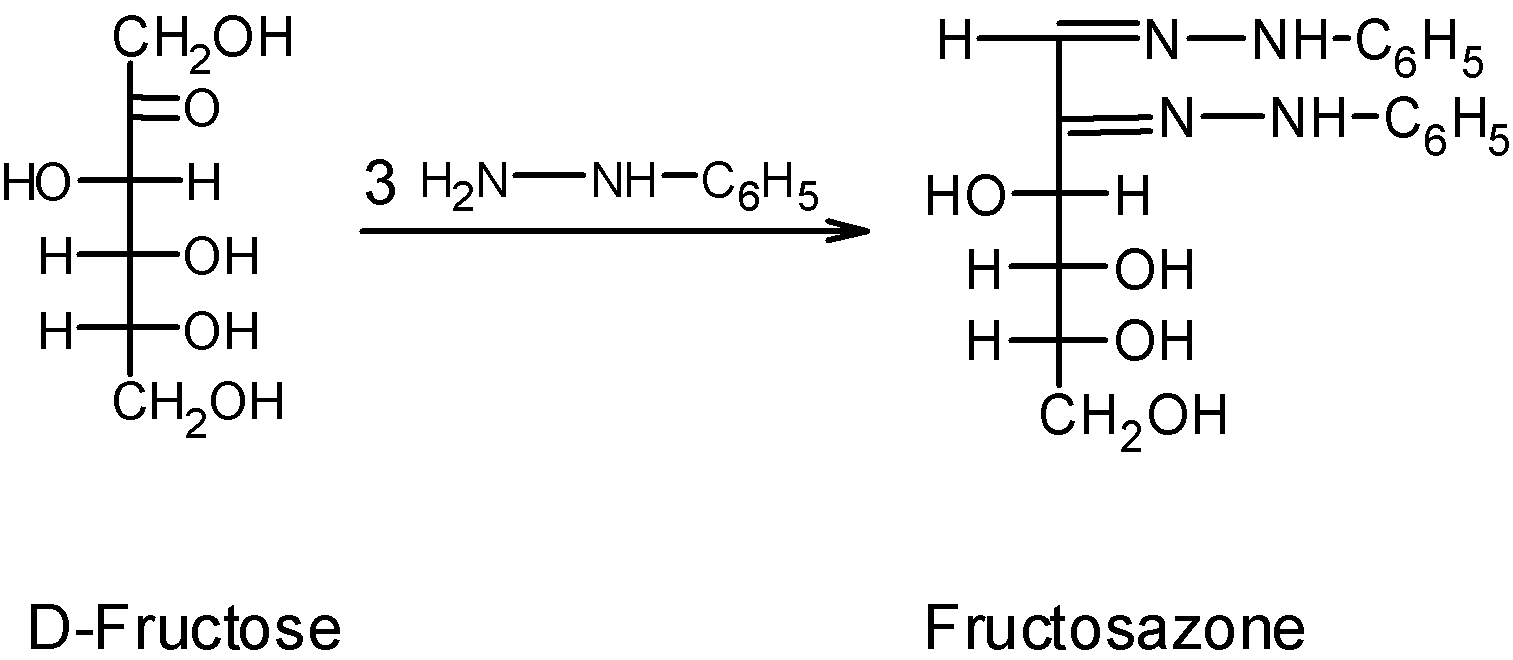
The aldose and ketose have the same molecular structure, except the position of the carbonyl group. In aldose, for example, glucose the carbonyl group is at the $\text{ }{{\text{C}}_{\text{1}}}\text{ }$position, and in ketose, for example, fructose the carbonyl group is situated at the $\text{ }{{\text{C}}_{2}}\text{ }$position. If we compare the osazone formation of glucose (aldose) and fructose (ketose) we observe that both utilized the three equivalents of the reagent but the product contains the two phenyl hydrazine residues. The one equivalent of reagent is utilized to oxidize the hydroxyl group to the carbonyl group. The adjacent $-\text{CHOH}$ group is oxidized. Thus, we can here say that the aldose and ketose have the same osazone since they have the same structure at all carbons accept the $\text{ }{{\text{C}}_{\text{1}}}\text{ }$and$\text{ }{{\text{C}}_{2}}\text{ }$. For example, the glucose and fructose from the glucosazone, and fructosazone have a similar structure.
Hence, (A) is the correct option.
Note: The osazone formation takes place only in the excess amount of phenyl hydrazine. In the limited supply of phenyl hydrazine, the reaction proceeds with the formation of one glucose phenylhydrazine as shown below,
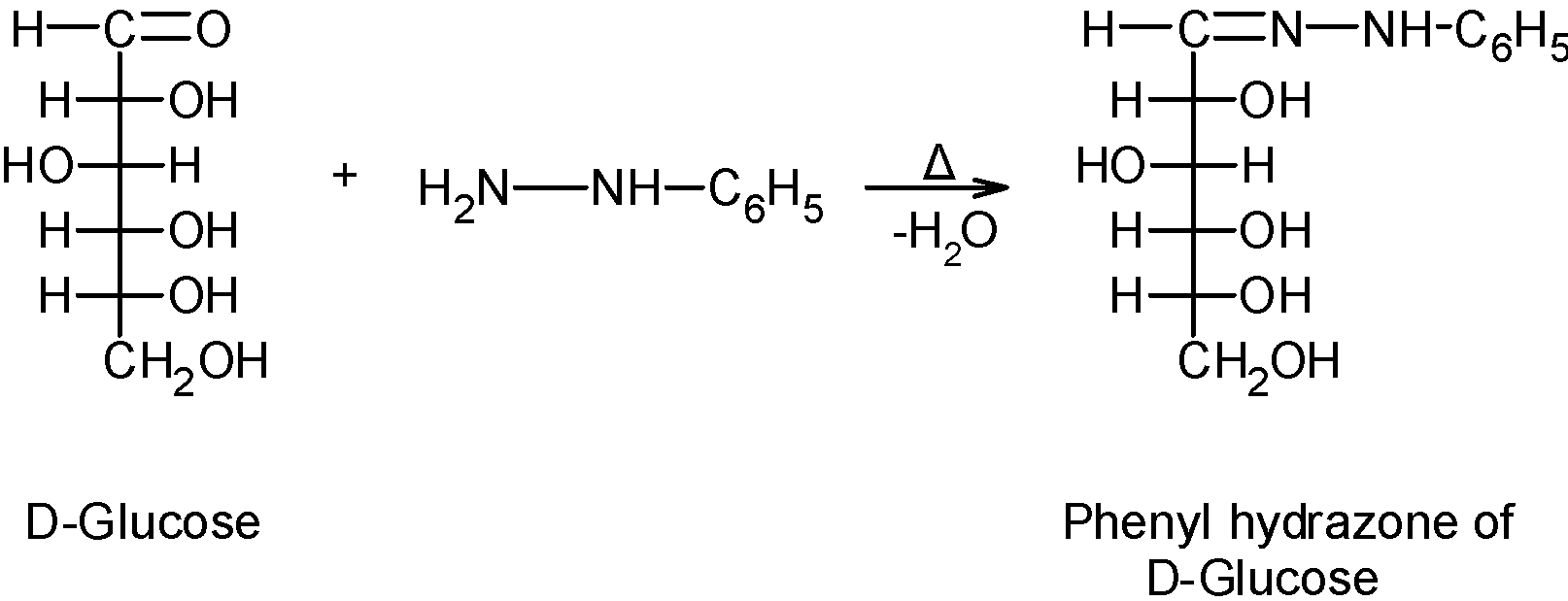
The phenyl hydrazine acts as an oxidizing agent. It oxidizes the hydroxyl group to the carbonyl group and itself reduces into aniline and ammonia.
\[\text{R}-\text{CHO}\xrightarrow{{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{NHN}{{\text{H}}_{\text{2}}}}\text{ R}-\text{CH}=\text{N}-\text{NH}-{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\]
Complete Step by step answer:
Sugar reacts with the excess of phenyl hydrazine to form phenyl hydrazone. This is called the osazone and the reaction is called the osazone formation reaction.
The aldehyde reacts with the [phenyl hydrazine to form phenyl hydrazine. However, the sugar which has aldehyde or ketone group, on warming up with the excess of phenylhydrazine reagent gives diphenyl hydrazones.
For example, let's consider that glucose reacts with the excess of phenylhydrazine to give the corresponding phenylosazone, which is glucosazone. The reaction takes place in three steps:
Step 1) The first molecule of phenyl hydrazine reacts with the aldehyde $\text{ }(-\text{CHO) }$group of the glucose to give the glucose phenylhydrazine. During this step, the one equivalent of the hydrazine is utilized to generate phenyl hydrazine.
Step 2) In step 2, the second molecule of the phenyl hydrazine oxidizes the reactive $\text{ }\alpha -\text{ }$hydroxyl group to generate the carbonyl group. Since, the aldehyde has already reacted to give hydrazine, the next equivalent of reagent attacks on the hydroxyl group on the adjacent carbon atom, oxidizes it to the carbonyl group.
Step 3) finally, the third molecule of the phenyl hydrazine reacts with the earlier formed carbonyl group and converts into hydrazine. Now, the glucose molecules have two phenyl hydrazone groups thus it is called the diphenyl hydrazine or glucosazone.
The progress of the reaction is as shown below,

Similarly, let's consider an example of fructose. The fructose undergoes the osazone formation as follows:

The aldose and ketose have the same molecular structure, except the position of the carbonyl group. In aldose, for example, glucose the carbonyl group is at the $\text{ }{{\text{C}}_{\text{1}}}\text{ }$position, and in ketose, for example, fructose the carbonyl group is situated at the $\text{ }{{\text{C}}_{2}}\text{ }$position. If we compare the osazone formation of glucose (aldose) and fructose (ketose) we observe that both utilized the three equivalents of the reagent but the product contains the two phenyl hydrazine residues. The one equivalent of reagent is utilized to oxidize the hydroxyl group to the carbonyl group. The adjacent $-\text{CHOH}$ group is oxidized. Thus, we can here say that the aldose and ketose have the same osazone since they have the same structure at all carbons accept the $\text{ }{{\text{C}}_{\text{1}}}\text{ }$and$\text{ }{{\text{C}}_{2}}\text{ }$. For example, the glucose and fructose from the glucosazone, and fructosazone have a similar structure.
Hence, (A) is the correct option.
Note: The osazone formation takes place only in the excess amount of phenyl hydrazine. In the limited supply of phenyl hydrazine, the reaction proceeds with the formation of one glucose phenylhydrazine as shown below,
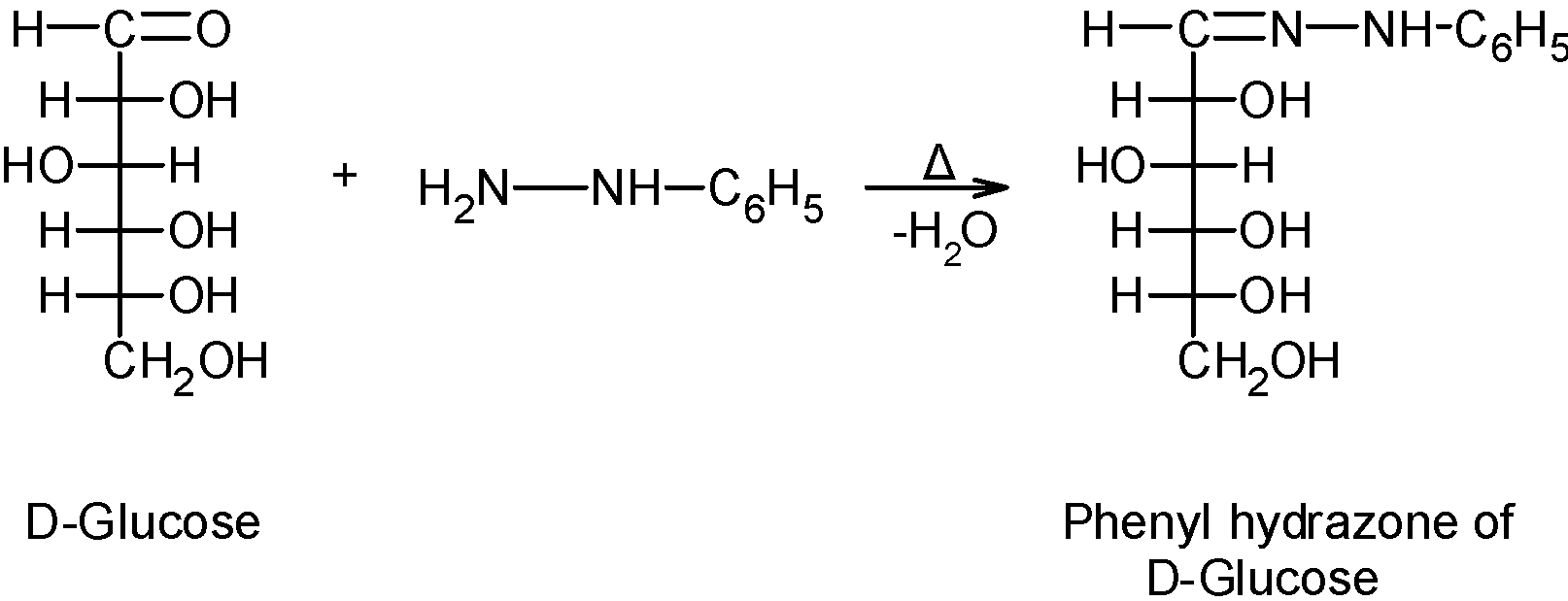
The phenyl hydrazine acts as an oxidizing agent. It oxidizes the hydroxyl group to the carbonyl group and itself reduces into aniline and ammonia.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




