
Which of the following possess the minimum boiling point:
A. 1- Pentyne
B. 1- Butyne
C. n-Butane
D. Isobutane
Answer
566.7k+ views
Hint: Alkane molecules are entirely held together by covalent bonds. These bonds either join two atoms of the same kind (carbons) and hence are non-polar, or join two atoms (C and H) that differ very little in electronegativity and hence are almost non-polar. Furthermore, slight bond polarities cancel out due to the symmetrical nature of alkane molecules is nonpolar. van der Waals forces holding non-polar molecules of alkanes together are weak and very short range. Thus, alkanes have lower boiling points. The intermolecular forces increase with the size of the molecule due to the increase in surface area. The process of boiling requires overcoming the intermolecular attractive forces and thus the boiling point increases with the increase in size.
Branched-chain isomers have lower boiling points as compared to straight-chain isomers. This is because branching the shape of the molecule tends to become spherical and thus, with branching surface area decreases.
Alkenes have higher boiling points than the corresponding alkanes. This is attributed to the stronger attractive forces in these compounds. This is because the \[\pi \]- electrons in the unsaturated hydrocarbons can be polarized more easily. This permits the formation of induced dipoles that are responsible for intermolecular attractive forces.
The boiling points of alkynes are slightly higher than those of the corresponding alkanes and alkenes. This is presumably due to the greater polarity of bonds in the case of alkynes. Boiling points increase regularly with the increase of molecular weights. This regular increase is due to the increasing van der Waals forces with the increasing size of the molecules.
Complete step by step answer:
The first option is 1- Pentyne. The structure of 1- Pentyne is
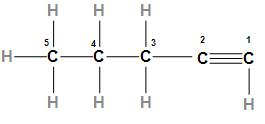
In the above structure, we can see 1- Pentyne is an alkyne that has five carbon atoms. As we know alkyne has high boiling points so 1- Pentyne will have a maximum boiling point.
The second option is 1- Butyne. The structure of 1- Butyne is
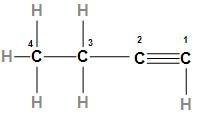
In the above structure, we can see that 1- Butyne is an alkyne with four carbon atoms. As we know that alkynes have high boiling points so 1- Butyne will also have a high boiling point which is less than the boiling point of 1- Pentyne (boiling point increases with the increase in the size of the carbon chain).
The third option is n-butane which is also called normal butane or butane. The structure of n-butane is
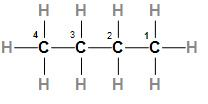
In the above structure, we can see that n-butane is a normal alkane with four carbon atoms. As we know that alkanes have a low boiling point in comparison to alkanes and alkynes so n-butane will have low boiling points.
The fourth option is Isobutane. The structure of Isobutane is
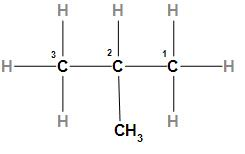
In the above structure, we can see that Isobutane is a branched-chain alkane with one methyl group attached to the second carbon. As we know that branched-chain alkane has a lower boiling point in comparison to straight chain, alkane, alkene, and alkyne so Isobutane has a minimum boiling point among all the compounds given in the option.
The order of the boiling points of the compounds in the given question is
1-Pentyne \[ > \] 1- Butyne \[ > \] n- Butane \[ > \] Isobutane
After discussing we can conclude that Isobutane has a minimum boiling point.
So, the correct answer is Option D .
Note:
Alkane, Alkene, and alkynes are the hydrocarbons compounds. Hydrocarbons are compounds that are made up only of carbon and hydrogen.The general order of the boiling for the hydrocarbons are
Alkyne \[ > \] Alkene \[ > \] Alkane \[ > \] Branched chain Alkene
The boiling point generally rises by about 20 to 30 degrees for the addition of each \[C{H_2}\] group.
Branched-chain isomers have lower boiling points as compared to straight-chain isomers. This is because branching the shape of the molecule tends to become spherical and thus, with branching surface area decreases.
Alkenes have higher boiling points than the corresponding alkanes. This is attributed to the stronger attractive forces in these compounds. This is because the \[\pi \]- electrons in the unsaturated hydrocarbons can be polarized more easily. This permits the formation of induced dipoles that are responsible for intermolecular attractive forces.
The boiling points of alkynes are slightly higher than those of the corresponding alkanes and alkenes. This is presumably due to the greater polarity of bonds in the case of alkynes. Boiling points increase regularly with the increase of molecular weights. This regular increase is due to the increasing van der Waals forces with the increasing size of the molecules.
Complete step by step answer:
The first option is 1- Pentyne. The structure of 1- Pentyne is

In the above structure, we can see 1- Pentyne is an alkyne that has five carbon atoms. As we know alkyne has high boiling points so 1- Pentyne will have a maximum boiling point.
The second option is 1- Butyne. The structure of 1- Butyne is

In the above structure, we can see that 1- Butyne is an alkyne with four carbon atoms. As we know that alkynes have high boiling points so 1- Butyne will also have a high boiling point which is less than the boiling point of 1- Pentyne (boiling point increases with the increase in the size of the carbon chain).
The third option is n-butane which is also called normal butane or butane. The structure of n-butane is

In the above structure, we can see that n-butane is a normal alkane with four carbon atoms. As we know that alkanes have a low boiling point in comparison to alkanes and alkynes so n-butane will have low boiling points.
The fourth option is Isobutane. The structure of Isobutane is

In the above structure, we can see that Isobutane is a branched-chain alkane with one methyl group attached to the second carbon. As we know that branched-chain alkane has a lower boiling point in comparison to straight chain, alkane, alkene, and alkyne so Isobutane has a minimum boiling point among all the compounds given in the option.
The order of the boiling points of the compounds in the given question is
1-Pentyne \[ > \] 1- Butyne \[ > \] n- Butane \[ > \] Isobutane
After discussing we can conclude that Isobutane has a minimum boiling point.
So, the correct answer is Option D .
Note:
Alkane, Alkene, and alkynes are the hydrocarbons compounds. Hydrocarbons are compounds that are made up only of carbon and hydrogen.The general order of the boiling for the hydrocarbons are
Alkyne \[ > \] Alkene \[ > \] Alkane \[ > \] Branched chain Alkene
The boiling point generally rises by about 20 to 30 degrees for the addition of each \[C{H_2}\] group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




