
Which of the following reactions represent the major product?
A.
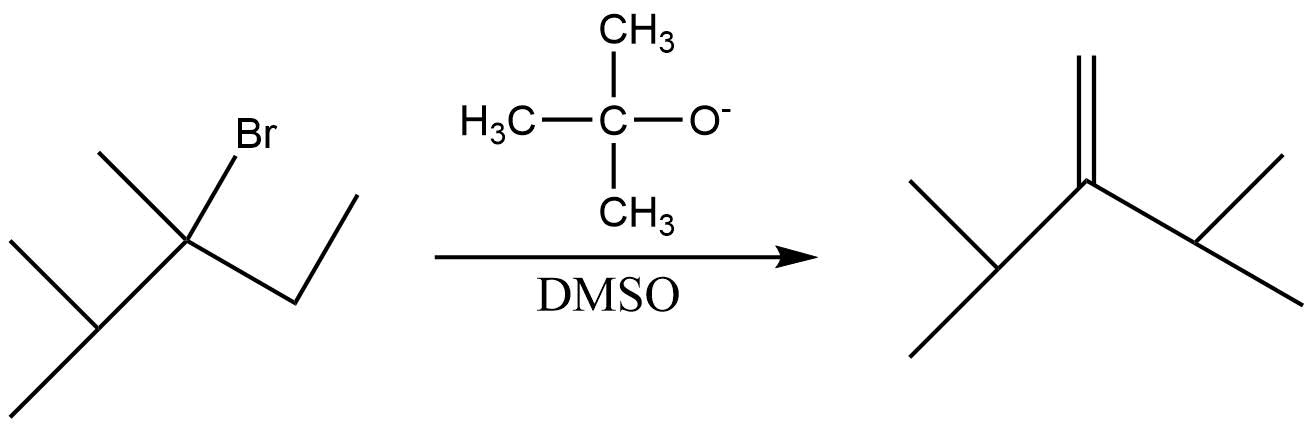
B.
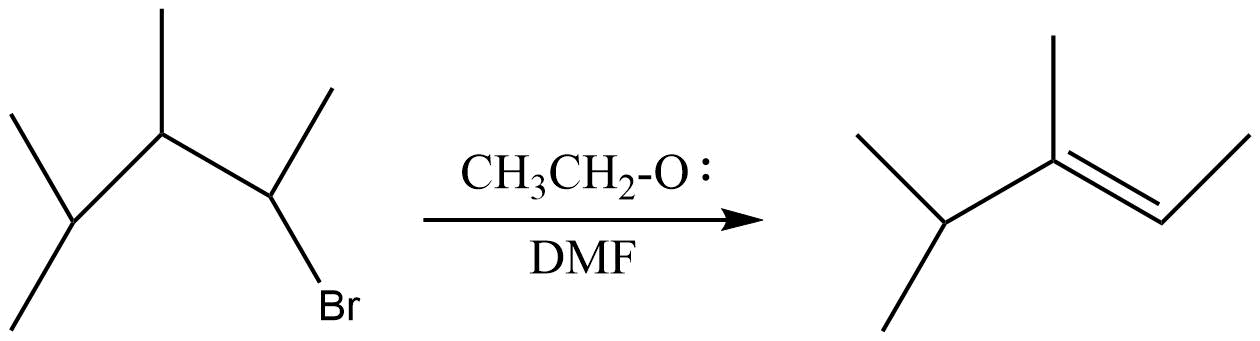
C.
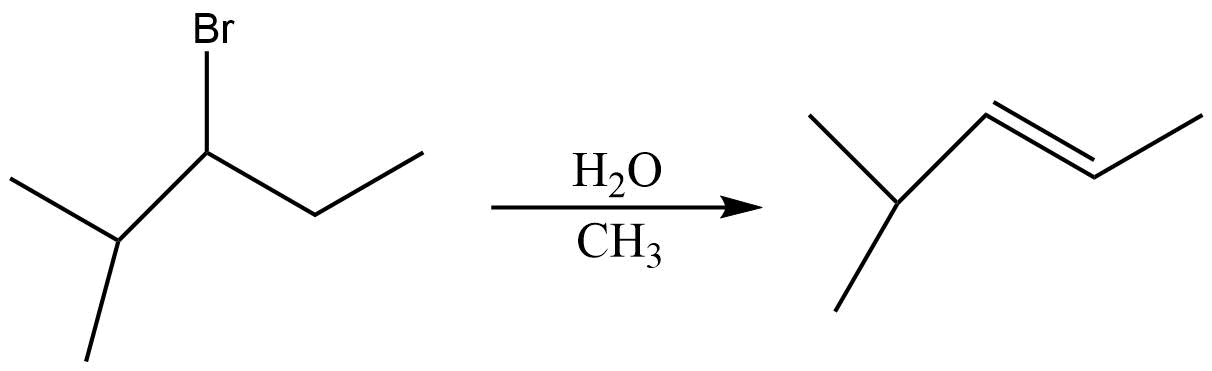
D.
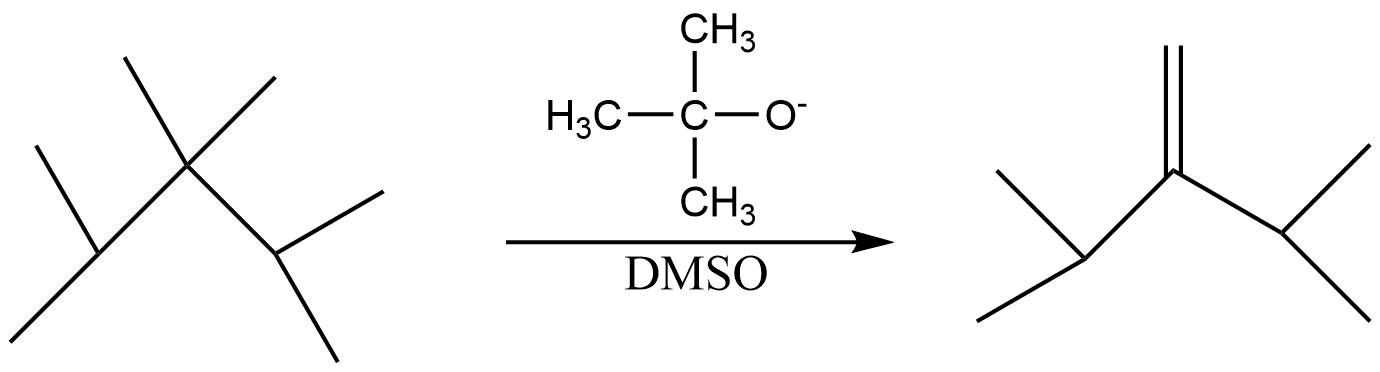




Answer
577.5k+ views
Hint: In this question, we are going to discuss the different alkyl halide reactions . Alkyl halides is the common name for haloalkanes . When one or more hydrogen atoms in a carbon chain are replaced with a halogen atom then the compound is termed as haloalkane .
Complete step by step answer:
In the above given reactions dehydrohalogenation takes place . Dehydrohalogentaion reaction is a reaction in which removal of $\alpha $ - halogen and $\beta $ - hydrogen takes place .
The major product is decided on the basis of Saytzeff rule . It states that in dehydrohalogenation reaction, the major product and more preferred alkene is the one having greater number of alkyl groups attached to double bonded carbon atoms .
However if the alkyl halide has a bulky alkyl group (such as tert-butyl group) or if the base used is bulky (such as tert-butoxide ion), then the less substituted product formed is the major product.
(A) In the first reaction tertiary alkyl halide reacts with dehydrohalogenation reagent . Since the alkyl group is bulky so the product formed is less substituted alkene .
(B) In the second reaction neither the base nor the alkyl group is bulky . So the product will be formed in accordance with Saytzeff rule , so more substituted alkene is formed .
(C) In the third reaction also neither the base nor the alkyl group is bulky . So the product will be formed in accordance with Saytzeff rule , so more substituted alkene is formed .
(D) In the fourth reaction both the base and the alkyl halide is bulky . Hence the less substituted alkene will be the major product .
Thus, the major product is represented in reaction A , B and D .
So, the correct option is (A), (B) and (D).
Note:
In the second reaction a secondary carbocation is formed which can rearrange itself to tertiary carbocation through 1,2 - hydride shift and formation of $2,3 - dimethyl - 2 - pentene$ takes place which is the major product .
Complete step by step answer:
In the above given reactions dehydrohalogenation takes place . Dehydrohalogentaion reaction is a reaction in which removal of $\alpha $ - halogen and $\beta $ - hydrogen takes place .
The major product is decided on the basis of Saytzeff rule . It states that in dehydrohalogenation reaction, the major product and more preferred alkene is the one having greater number of alkyl groups attached to double bonded carbon atoms .
However if the alkyl halide has a bulky alkyl group (such as tert-butyl group) or if the base used is bulky (such as tert-butoxide ion), then the less substituted product formed is the major product.
(A) In the first reaction tertiary alkyl halide reacts with dehydrohalogenation reagent . Since the alkyl group is bulky so the product formed is less substituted alkene .
(B) In the second reaction neither the base nor the alkyl group is bulky . So the product will be formed in accordance with Saytzeff rule , so more substituted alkene is formed .
(C) In the third reaction also neither the base nor the alkyl group is bulky . So the product will be formed in accordance with Saytzeff rule , so more substituted alkene is formed .
(D) In the fourth reaction both the base and the alkyl halide is bulky . Hence the less substituted alkene will be the major product .
Thus, the major product is represented in reaction A , B and D .
So, the correct option is (A), (B) and (D).
Note:
In the second reaction a secondary carbocation is formed which can rearrange itself to tertiary carbocation through 1,2 - hydride shift and formation of $2,3 - dimethyl - 2 - pentene$ takes place which is the major product .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




