
Which of the following solids is the structure of \[CsCl\] crystal?
A. Body-centered cubic
B. Simple cubic
C. Face centered cubic
D. Edge centered cubic
Answer
594k+ views
Hint: We must know about crystal structure. We can define crystal structure as the ordered arrangement of atoms, ions, or molecules in a crystalline material. Therefore, \[CsCl\] has a specific crystal structure. \[CsCl\] consists of \[Cs\] being at centre and surrounded by 8 atoms of \[Cl\]
Complete answer:
Let’s start with discussing the options that are given to us for a better understanding.
The first one is body-centred cubic, the body-centred cubic consists of body centred unit cell. In these, there are eight atoms at each corner of the cube and each atom is shared by 8 unit cells and one central atom is there.
The second one is simple cubic, the simple cubic consists of a simple cubic unit cell in which there are only eight atoms at each corner of the cube and each atom is shared by 8 other unit cells.
The third one is Face centred cubic which consists of a face-centred unit cell in these 8 atoms are at the corners of the cube and each atom is shared by 8 other unit cells and one atom each at the faces and each atom is shared by 2 unit cell so a total of 6 atoms.
The last one is Edge centred cubic which consists of Edge centred unit cells. In these there are atoms which are shared by 4 other unit cells.
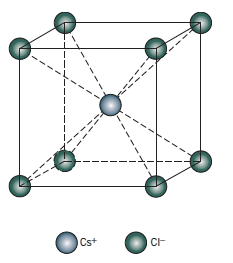
In case of \[CsCl\], the Cs stays at the centre and is surrounded by 8 Cl atoms which form Body centred cubic structure.
So, the answer to this question is A. Body centred cubic structure.
Note:
These structures tell not only about how these atoms are being placed but also about how compact the structure is, how many void spaces are there or how much percentage of the structure is being covered by the atoms and how much is empty. These structures tell the characteristic of the molecule as how the molecule will react or which category it belongs to.
Complete answer:
Let’s start with discussing the options that are given to us for a better understanding.
The first one is body-centred cubic, the body-centred cubic consists of body centred unit cell. In these, there are eight atoms at each corner of the cube and each atom is shared by 8 unit cells and one central atom is there.
The second one is simple cubic, the simple cubic consists of a simple cubic unit cell in which there are only eight atoms at each corner of the cube and each atom is shared by 8 other unit cells.
The third one is Face centred cubic which consists of a face-centred unit cell in these 8 atoms are at the corners of the cube and each atom is shared by 8 other unit cells and one atom each at the faces and each atom is shared by 2 unit cell so a total of 6 atoms.
The last one is Edge centred cubic which consists of Edge centred unit cells. In these there are atoms which are shared by 4 other unit cells.

In case of \[CsCl\], the Cs stays at the centre and is surrounded by 8 Cl atoms which form Body centred cubic structure.
So, the answer to this question is A. Body centred cubic structure.
Note:
These structures tell not only about how these atoms are being placed but also about how compact the structure is, how many void spaces are there or how much percentage of the structure is being covered by the atoms and how much is empty. These structures tell the characteristic of the molecule as how the molecule will react or which category it belongs to.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




