
Which of the following species/ compounds is not hypo valent?
A. \[C{H_3}^ + \]
B. \[{B_2}{H_6}\]
C. \[N{H_2}^ + \]
D. \[Al{F_3}\]
Answer
583.5k+ views
Hint: There are certain anomalies observed while the formation of certain compounds. There are certain cases where the number of electrons on the central atom is less than 8. This does not comply with the octet rule. But even then, the compounds formed are stable. Such compounds are known as Hypo valent compounds.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Now, to determine which of the given compounds are hypo valent in nature, we draw and observe their molecular structures
\[C{H_3}^ + \]
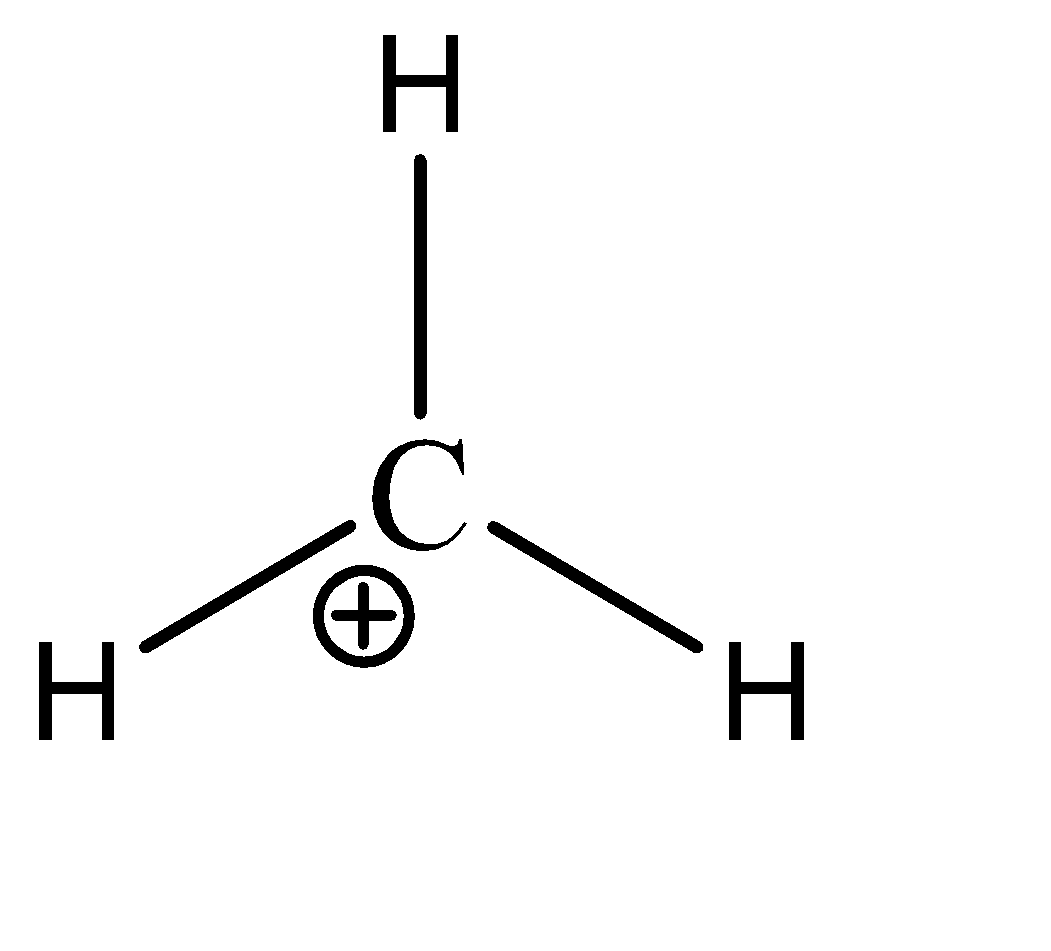
We can see that there are 3 sigma bonds and no lone pairs on the central atom. Hence there are 6 electrons present in the valence shell of the carbon. Hence, the given compound is hypo valent.
\[{B_2}{H_6}\]
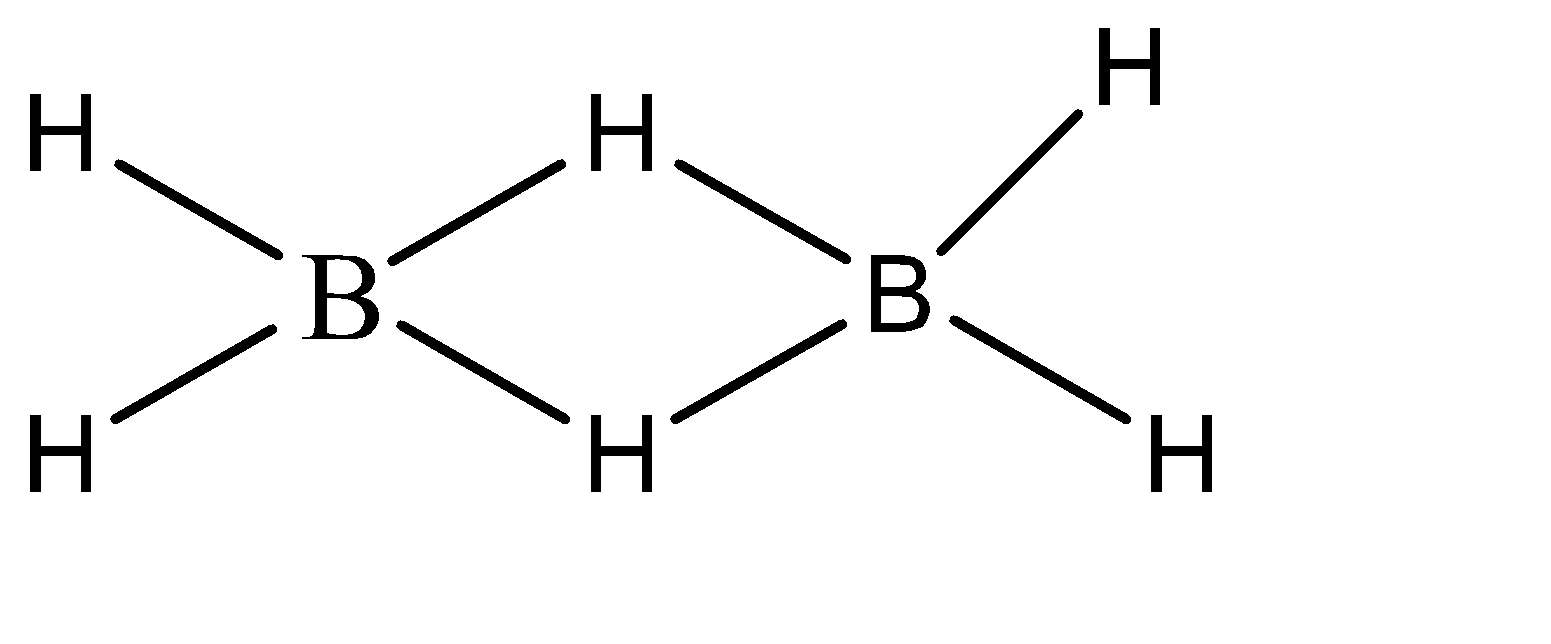
The number of electrons present in the valence shell of a boron atom is 3. Now, 4 bonds are formed on each boron atom. Hence, the total number of electrons in the valence shell of boron is 7. Hence, the given compound is hypo valent.
\[N{H_2}^ + \]
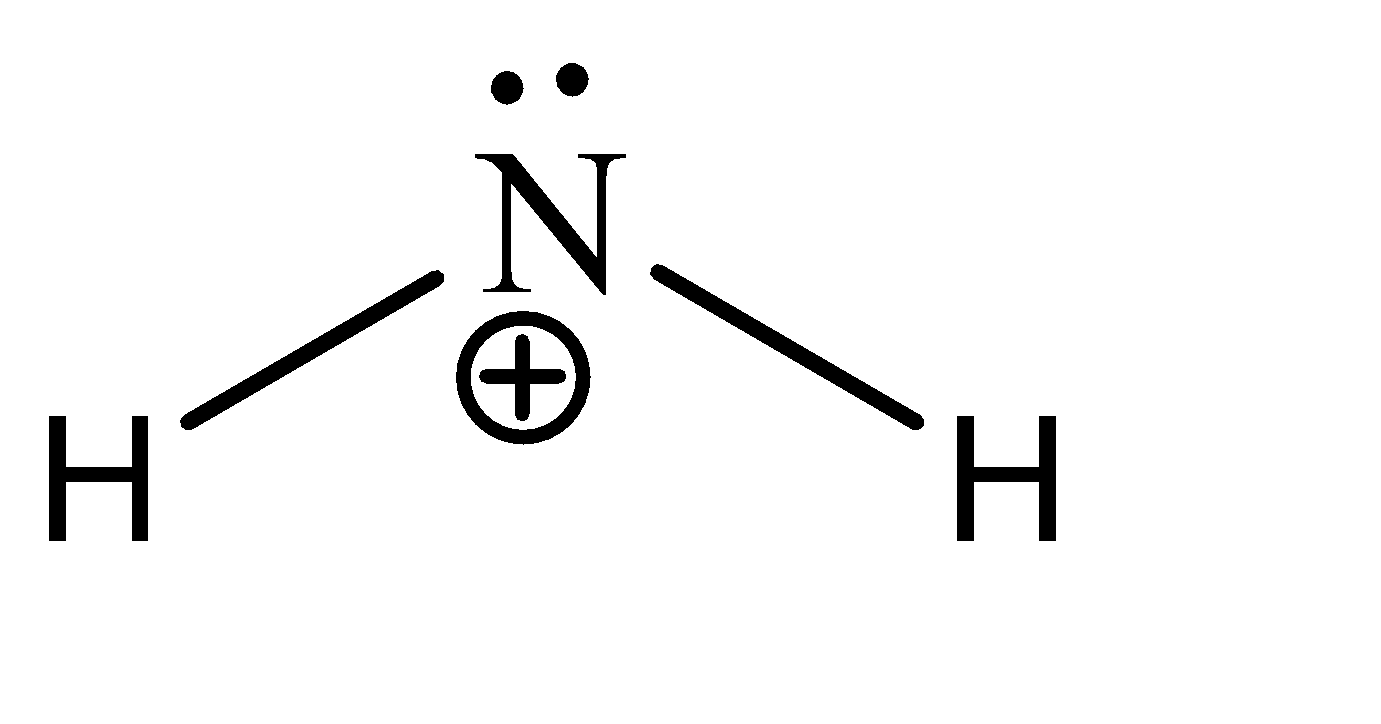
We can see that there are 2 sigma bonds and 1 lone pair on the central atom. Hence there are 6 electrons present in the valence shell of the carbon. Hence, the given compound is hypo valent.
\[Al{F_3}\]
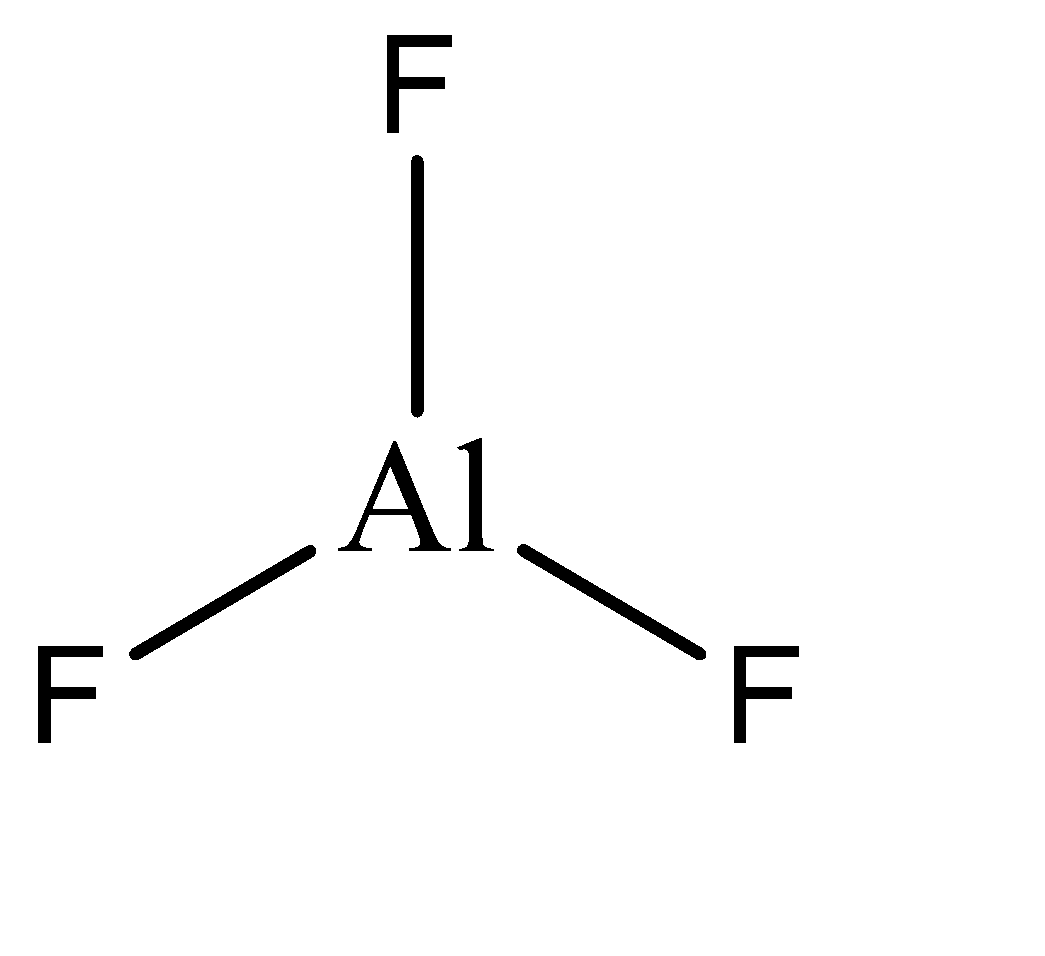
\[Al{F_3}\] is ionic solid, so it is formed by transfer of electrons from one atom to another. Al has an electronic configuration of \[1{s^2}2{s^2}2{p^8}3{s^3}\] and hence tends to lose the 3 electrons in its valence shell, which are taken by the fluorine atoms. Hence the final electronic configuration of Al in \[Al{F_3}\] is \[1{s^2}2{s^2}2{p^8}\] . Hence, the given compound is not hypo valent.
Hence, Option D is the correct option
Note: On the other hand, there exist certain compounds where the central atom has more than 8 electrons in its valence shell. Such compounds are known as hypervalent compounds. Some examples of hypervalent compounds are: \[PC{l_5}\] , \[S{F_6}\] , \[Cl{F_3}\] , \[{I_3}^ - \] , etc.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Now, to determine which of the given compounds are hypo valent in nature, we draw and observe their molecular structures
\[C{H_3}^ + \]

We can see that there are 3 sigma bonds and no lone pairs on the central atom. Hence there are 6 electrons present in the valence shell of the carbon. Hence, the given compound is hypo valent.
\[{B_2}{H_6}\]

The number of electrons present in the valence shell of a boron atom is 3. Now, 4 bonds are formed on each boron atom. Hence, the total number of electrons in the valence shell of boron is 7. Hence, the given compound is hypo valent.
\[N{H_2}^ + \]

We can see that there are 2 sigma bonds and 1 lone pair on the central atom. Hence there are 6 electrons present in the valence shell of the carbon. Hence, the given compound is hypo valent.
\[Al{F_3}\]

\[Al{F_3}\] is ionic solid, so it is formed by transfer of electrons from one atom to another. Al has an electronic configuration of \[1{s^2}2{s^2}2{p^8}3{s^3}\] and hence tends to lose the 3 electrons in its valence shell, which are taken by the fluorine atoms. Hence the final electronic configuration of Al in \[Al{F_3}\] is \[1{s^2}2{s^2}2{p^8}\] . Hence, the given compound is not hypo valent.
Hence, Option D is the correct option
Note: On the other hand, there exist certain compounds where the central atom has more than 8 electrons in its valence shell. Such compounds are known as hypervalent compounds. Some examples of hypervalent compounds are: \[PC{l_5}\] , \[S{F_6}\] , \[Cl{F_3}\] , \[{I_3}^ - \] , etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




