
Which of the following species is not electrophilic in nature:
a.) $\overset{+}{\mathop{Cl}}\,$
b.) $B{{H}_{3}}$
c.) ${{H}_{3}}\overset{+}{\mathop{O}}\,$
d.) $\overset{+}{\mathop{N}}\,{{O}_{2}}$
Answer
579k+ views
Hint: Electrophile are referred to as the electron loving species. Electrophiles are positive charge species and they are electron deficient. They bond with the electron-rich species which are known as nucleophiles.
Complete step by step answer:
Electrophiles accept electrons; they are the Lewis acids. Electrophiles are electron deficient; they have a partially positive charge which means that the atom does not have a complete octet.
In option first Chlorine has a positive charge. Chlorine has deficiency of electrons, it accepts an electron pair to complete its octet hence it behaves as an electrophile.
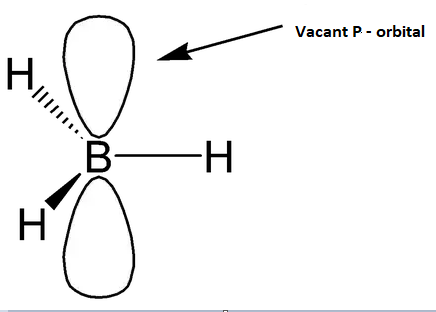
In option second boron has three hydrogen atoms attached to it. Boron is an electrophile due to the presence of an empty p orbital in boron.
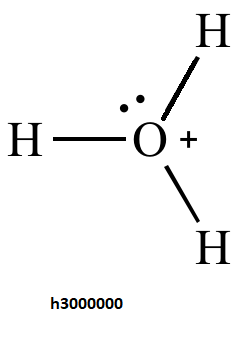
In option third oxygen atom binds with three hydrogen. Oxygen has 6 electrons in its valence shell but due to the presence of a positive charge oxygen will have 5 electrons in its valence shell. After forming a bond with three hydrogen there is still a lone pair of electrons left on the oxygen atom and due to the presence of positive charge it cannot donate the electron pair .Hence it will behave as a nucleophile.
In option four nitrogen is doubly bonded to two oxygen atoms. The valence electrons in nitrogen is 5 and due to the presence of a positive charge the valence electrons will be 4. As nitrogen does not have an octet around it will behave as an electrophile.
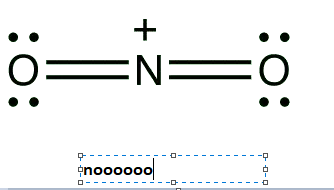
So, the correct answer is “Option C”.
Note: Electrophiles mostly react with nucleophiles. For example sulphur dioxide reacts with water which is a nucleophile to form Sulphuric acid. Electrophiles are electron- loving species while nucleophiles are nucleus loving species.
Complete step by step answer:
Electrophiles accept electrons; they are the Lewis acids. Electrophiles are electron deficient; they have a partially positive charge which means that the atom does not have a complete octet.
In option first Chlorine has a positive charge. Chlorine has deficiency of electrons, it accepts an electron pair to complete its octet hence it behaves as an electrophile.
In option second boron has three hydrogen atoms attached to it. Boron is an electrophile due to the presence of an empty p orbital in boron.

In option third oxygen atom binds with three hydrogen. Oxygen has 6 electrons in its valence shell but due to the presence of a positive charge oxygen will have 5 electrons in its valence shell. After forming a bond with three hydrogen there is still a lone pair of electrons left on the oxygen atom and due to the presence of positive charge it cannot donate the electron pair .Hence it will behave as a nucleophile.

In option four nitrogen is doubly bonded to two oxygen atoms. The valence electrons in nitrogen is 5 and due to the presence of a positive charge the valence electrons will be 4. As nitrogen does not have an octet around it will behave as an electrophile.
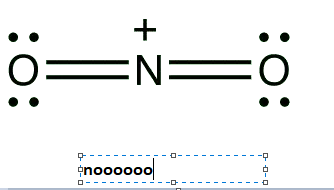
So, the correct answer is “Option C”.
Note: Electrophiles mostly react with nucleophiles. For example sulphur dioxide reacts with water which is a nucleophile to form Sulphuric acid. Electrophiles are electron- loving species while nucleophiles are nucleus loving species.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




