
Which of the following statements is incorrect about xenon trioxide?
(A)It can be prepared by hydrolysis of $Xe{F_4}$
(B)It can be prepared by hydrolysis of $Xe{F_6}$
(C)It is a non volatile solid
(D)It’s aqueous is slightly basic
Answer
524.7k+ views
Hint :Xenon trioxide is a compound of the element xenon. In this compound, the oxidation state of xenon is $ + 6$. It is unstable in nature. Xenon trioxide acts as a powerful oxidizing agent. The chemical formula of xenon trioxide is $Xe{O_3}$. It is colourless and is a crystalline solid. If xenon trioxide comes into contact with any organic material, it becomes dangerously explosive.
Complete Step By Step Answer:
The structure of xenon trioxide is as shown below:
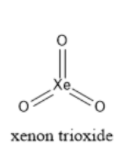
Xenon trioxide is trigonal pyramidal in shape. This is due to the presence of lone pairs. Xenon trioxide also violates the octet rule as it is present in the fifth row of the periodic table. When this compound is in water, it liberates oxygen slowly. Xenon trioxide is a non-volatile solid. It’s aqueous is not basic. If $Xe{F_6}$ undergoes complete hydrolysis, it produces xenon trioxide. The reaction is given below:
$Xe{F_6} + 3{H_2}O \to Xe{O_3} + 6HF$
If $Xe{F_4}$undergoes hydrolysis in the presence of excess of water, it produces xenon trioxide. The hydrolysis reaction is given below:
$3Xe{F_4} + 6{H_2}O \to 2Xe + Xe{O_3} + 12HF + \dfrac{3}{2}{O_2}$
From the above reactions, we can say that xenon trioxide can be prepared by hydrolysis of $Xe{F_4}$ and $Xe{F_6}$. And also, xenon trioxide is a non-volatile solid.
Therefore, Option D is the correct answer.
Note :
Xenon trioxide explodes on heating. So, xenon trioxide should be handled very carefully. Xenon trioxide is stable in dry air. But if it comes in contact with humid air, it absorbs water and forms a concentrated solution. The crystal structure of xenon trioxide is orthorhombic.
Complete Step By Step Answer:
The structure of xenon trioxide is as shown below:

Xenon trioxide is trigonal pyramidal in shape. This is due to the presence of lone pairs. Xenon trioxide also violates the octet rule as it is present in the fifth row of the periodic table. When this compound is in water, it liberates oxygen slowly. Xenon trioxide is a non-volatile solid. It’s aqueous is not basic. If $Xe{F_6}$ undergoes complete hydrolysis, it produces xenon trioxide. The reaction is given below:
$Xe{F_6} + 3{H_2}O \to Xe{O_3} + 6HF$
If $Xe{F_4}$undergoes hydrolysis in the presence of excess of water, it produces xenon trioxide. The hydrolysis reaction is given below:
$3Xe{F_4} + 6{H_2}O \to 2Xe + Xe{O_3} + 12HF + \dfrac{3}{2}{O_2}$
From the above reactions, we can say that xenon trioxide can be prepared by hydrolysis of $Xe{F_4}$ and $Xe{F_6}$. And also, xenon trioxide is a non-volatile solid.
Therefore, Option D is the correct answer.
Note :
Xenon trioxide explodes on heating. So, xenon trioxide should be handled very carefully. Xenon trioxide is stable in dry air. But if it comes in contact with humid air, it absorbs water and forms a concentrated solution. The crystal structure of xenon trioxide is orthorhombic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




