
Which of the following statements is true for X?
\[Phenol + CC{l_4} + KOH \to X;\]
A) It gives effervescence with \[NaHC{O_3}\]
B) Gives silver mirror with Tollen's reagent
C) Does not give the red colour with $FeC{l_3}$
D) All of the above
Answer
565.5k+ views
Hint: The given reaction known as the Reimer-Tiemann reaction.
Reimer Tiemann reaction is a kind of a substitution reaction. It is popularly named after two chemists namely Karl Reimer and Ferdinand Tiemann. The reaction is known for the ortho-formylation of phenols.
Complete step by step answer:
When phenols i.e. \[{C_6}{H_5}OH\] are treated with \[CHC{l_3}\] (chloroform) in the presence of \[NaOH\] (sodium hydroxide), an aldehyde group \[\left( { - CHO} \right)\] is introduced at the ortho position of benzene ring leading to the formation of o-hydroxybenzaldehyde. The reaction is referred to as the Reimer Tiemann reaction. (a) Salicylic acid creates effervescence with sodium bicarbonate or \[NaHC{O_3}\] which is a characteristic feature of carboxylic acid. Therefore, it ensures the presence of a carboxylic group in it.
The reaction is as follows:
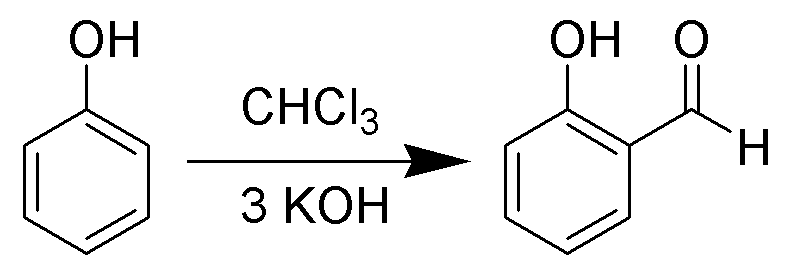
(b) It does not produce a silver mirror with Tollen's reagent because it does not have an aldehyde group.
(c) It gives red colour with the neutral \[FeC{l_3}\] as it consists of an enol group i.e., \[ = \mid C - OH\;\] group.
So, the correct answer is Option A.
Note: The Reimer–Tiemann reaction chemical reaction is used for the ortho-formylation of phenols with the simplest example being the conversion of phenol to salicylaldehyde. The reaction is highly exothermic. The mechanism starts with abstraction of the proton from chloroform with the base to form a trichlorocarban ion. It spontaneously loses a chloride ion to form a neutral dichlorocarbene. The base even deprotonates the phenol reagent which further attacks the carbene. A sequence of steps and a final acid work up result in formation of o-hydroxy benzaldehyde product.
Reimer Tiemann reaction is a kind of a substitution reaction. It is popularly named after two chemists namely Karl Reimer and Ferdinand Tiemann. The reaction is known for the ortho-formylation of phenols.
Complete step by step answer:
When phenols i.e. \[{C_6}{H_5}OH\] are treated with \[CHC{l_3}\] (chloroform) in the presence of \[NaOH\] (sodium hydroxide), an aldehyde group \[\left( { - CHO} \right)\] is introduced at the ortho position of benzene ring leading to the formation of o-hydroxybenzaldehyde. The reaction is referred to as the Reimer Tiemann reaction. (a) Salicylic acid creates effervescence with sodium bicarbonate or \[NaHC{O_3}\] which is a characteristic feature of carboxylic acid. Therefore, it ensures the presence of a carboxylic group in it.
The reaction is as follows:

(b) It does not produce a silver mirror with Tollen's reagent because it does not have an aldehyde group.
(c) It gives red colour with the neutral \[FeC{l_3}\] as it consists of an enol group i.e., \[ = \mid C - OH\;\] group.
So, the correct answer is Option A.
Note: The Reimer–Tiemann reaction chemical reaction is used for the ortho-formylation of phenols with the simplest example being the conversion of phenol to salicylaldehyde. The reaction is highly exothermic. The mechanism starts with abstraction of the proton from chloroform with the base to form a trichlorocarban ion. It spontaneously loses a chloride ion to form a neutral dichlorocarbene. The base even deprotonates the phenol reagent which further attacks the carbene. A sequence of steps and a final acid work up result in formation of o-hydroxy benzaldehyde product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




