
Which of the following structures is similar to graphite?
\[
A.BN \\
B.B \\
C.{B_4}C \\
D.{B_2}{H_6} \\
\]
Answer
591.6k+ views
Hint: We must remember that the carbon atoms present in graphite are \[\;sp2\] hybridized and are bonded to each other in such a way that 3 out of the 4 valences of carbon gets bonded to another present in the same plane.
Complete step by step answer:
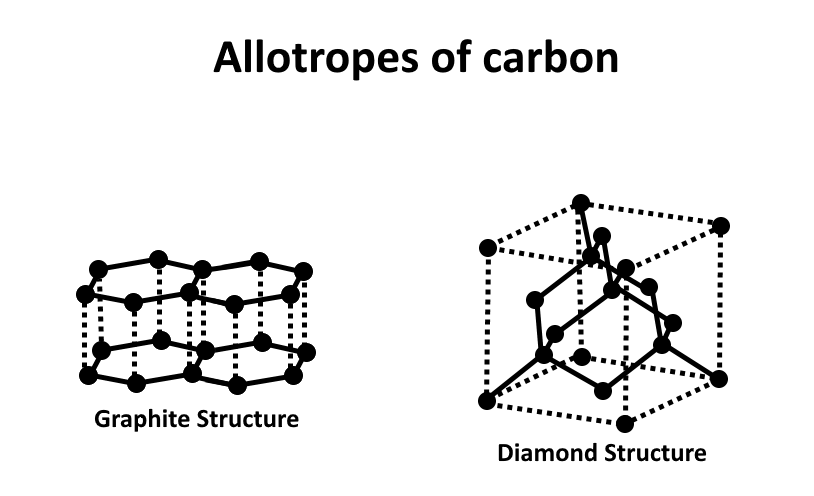
To answer this question we need to understand the basic structure of graphite. Graphite is one of the allotropes of carbon.
The carbon atoms present in graphite are \[\;sp2\] hybridized and are bonded to each other in such a way that 3 out of the 4 valences of carbon gets bonded to another present in the same plane.
This interlinking forms a hexagonal structure in a 2-dimensional plane. In this structure, the bond angle that is formed between the carbon atoms is 120 degrees with a bond length in the plane of 1.421 angstroms.
So the structure that closely resembles that of the graphite will be \[BN\]
Boron nitride also comes in 2 forms namely hexagonal boron nitride and cubic boron nitride. But to keep the discussion short we will discuss the similarities of hexagonal boron nitride and graphite.
\[H - BN\] or \[BN\] structure consists of an alternate arrangement of Boron and Nitrogen atoms in a 2-dimensional plane and in turn, forms a hexagonal ring structure. The N and B atoms are combined by an \[\;sp2\] orbital forming a strong sigma bond.
Due to the formation of hexagonal rings, they share a common structure but with different atoms.
Hence the correct answer will be A. \[BN\]
Note:
We must know that graphite is one of the allotropes of carbon and has the most different properties compared to other allotropes like coal and diamond. Graphite is able to conduct electricity as one of the electrons in each carbon of hexagon is free and roams in the lattice and hence conducts electricity.
Complete step by step answer:
To answer this question we need to understand the basic structure of graphite. Graphite is one of the allotropes of carbon.
The carbon atoms present in graphite are \[\;sp2\] hybridized and are bonded to each other in such a way that 3 out of the 4 valences of carbon gets bonded to another present in the same plane.
This interlinking forms a hexagonal structure in a 2-dimensional plane. In this structure, the bond angle that is formed between the carbon atoms is 120 degrees with a bond length in the plane of 1.421 angstroms.
So the structure that closely resembles that of the graphite will be \[BN\]

Boron nitride also comes in 2 forms namely hexagonal boron nitride and cubic boron nitride. But to keep the discussion short we will discuss the similarities of hexagonal boron nitride and graphite.
\[H - BN\] or \[BN\] structure consists of an alternate arrangement of Boron and Nitrogen atoms in a 2-dimensional plane and in turn, forms a hexagonal ring structure. The N and B atoms are combined by an \[\;sp2\] orbital forming a strong sigma bond.
Due to the formation of hexagonal rings, they share a common structure but with different atoms.
Hence the correct answer will be A. \[BN\]
Note:
We must know that graphite is one of the allotropes of carbon and has the most different properties compared to other allotropes like coal and diamond. Graphite is able to conduct electricity as one of the electrons in each carbon of hexagon is free and roams in the lattice and hence conducts electricity.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




