
Which of the following vitamins has isoprene units in its structure:
(A) Vitamin $ A $
(B) Vitamin $ C $
(C) Vitamin $ {B_2} $
(D) Vitamin $ D $
Answer
559.5k+ views
Hint: Vitamins are referred to as the organic compounds which are required in our body in a small amount with our diet to maintain our health and growth in a specific bio-system. Vitamins are classified into different groups. We will figure out the vitamin which has an isoprene unit in its structure.
Complete step by step answer
Now we have some basic understanding of vitamins and their importance in our body. The vitamins are classified as $ A,B,C,D,E $ and many more. These groups may also have subgroups. Now we will go back to our question first. The questions say that we need to identify the vitamin which has isoprene units in its structure. So, now we will discuss isoprene then we will discuss the structure of vitamin. So, isoprene is a chemical organic compound that is an unsaturated hydrocarbon. The IUPAC name of isoprene is $ 2 - $ methyl- $ 1,3 - $ butadiene. The chemical structure of isoprene is given below.
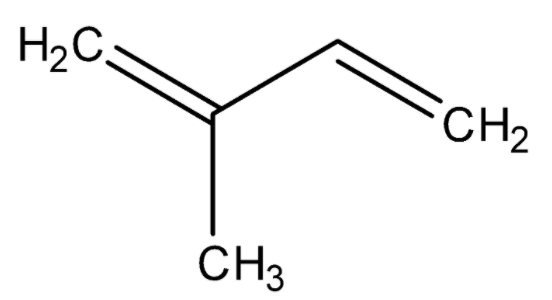
Now we are aware of the structure of isoprene. Now we will consider a vitamin and study its structure and then we will figure out the isoprene unit in its structure. So, let’s consider Vitamin $ A $ . The structure of vitamin $ A $ is given below.
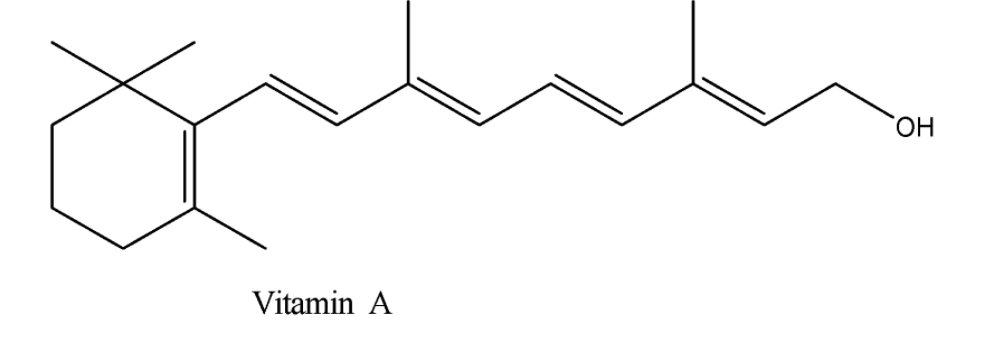
We can observe from the above structure that there are $ 4 $ isoprene units in the vitamin $ A $ . They are linked head to tail.
Therefore, the correct option is (A).
Note:
Vitamin $ A $ is commonly known as Retinol. Retinol is a fat-soluble vitamin and this vitamin is stored in the liver and adipose. The deficiency of Vitamin $ A $ causes night blindness and hardening of the cornea. The best source is carrot, milk, butter, and Cod liver oil.
Complete step by step answer
Now we have some basic understanding of vitamins and their importance in our body. The vitamins are classified as $ A,B,C,D,E $ and many more. These groups may also have subgroups. Now we will go back to our question first. The questions say that we need to identify the vitamin which has isoprene units in its structure. So, now we will discuss isoprene then we will discuss the structure of vitamin. So, isoprene is a chemical organic compound that is an unsaturated hydrocarbon. The IUPAC name of isoprene is $ 2 - $ methyl- $ 1,3 - $ butadiene. The chemical structure of isoprene is given below.

Now we are aware of the structure of isoprene. Now we will consider a vitamin and study its structure and then we will figure out the isoprene unit in its structure. So, let’s consider Vitamin $ A $ . The structure of vitamin $ A $ is given below.

We can observe from the above structure that there are $ 4 $ isoprene units in the vitamin $ A $ . They are linked head to tail.
Therefore, the correct option is (A).
Note:
Vitamin $ A $ is commonly known as Retinol. Retinol is a fat-soluble vitamin and this vitamin is stored in the liver and adipose. The deficiency of Vitamin $ A $ causes night blindness and hardening of the cornea. The best source is carrot, milk, butter, and Cod liver oil.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




