
Which of the following will not show mutarotation?
A) Maltose
B) Lactose
C) Glucose
D) Sucrose
Answer
598.5k+ views
Hint- we must remember that, only reducing sugars have a free aldehyde $( - CHO)$ or ketone \[\left( { > C = O} \right)\] group which are capable of showing mutarotation. Hence reducing sugars can undergo mutarotation and non-reducing sugars cannot undergo mutarotation.
Complete step by step answer:
We must understand that mutarotation is a process that changes the optical rotation of compounds in the aqueous solution. This is because of the change in the equipoise in between two anomers.
Let’s we discuss the structure of these options in detail
We have to remember that the maltose is a reducing sugar which has a hydroxyl group on the ring. It can exhibits mutarotation in aqueous solution, because the α and β-isomers that are formed by the different conformations of the anomeric carbon exhibit different specific rotations, and in aqueous solutions, these two forms are in equilibrium.
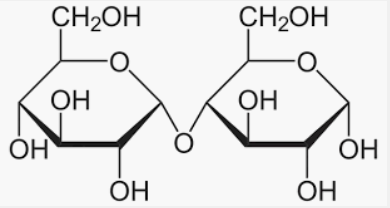
And we must know that the disaccharide sugar is lactose which is available in the daily product milk sugar. And it comprises one glucose molecule linked to a galactose molecule by an β(1→4)-glycosidic linkage. Hence, It is a reducing sugar and hence shows mutarotation.
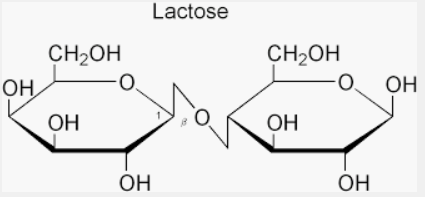
We must understand that the Sucrose does not show mutarotation. However, this property of mutarotation is not exhibited by all sugars. Sucrose does not have free aldehyde (-CHO) or ketone (>C=O) group. Therefore, sucrose is incapable of showing mutarotation.
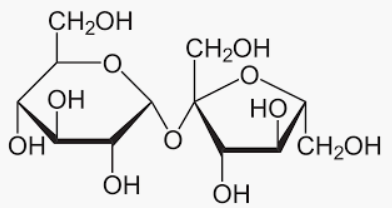
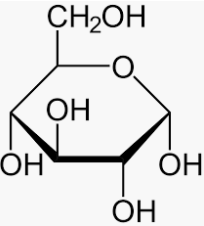
Glucose is also a reducing sugar show mutarotation.
We can conclude that the Sucrose is not a reducing sugar because it does not have a hydroxyl group in the ring. Hence, sucrose does not show mutarotation.
Note: We can also know that the cellulose also does not show mutarotation as sucrose .This is why because of hemiacetals (or hemiketals). They do not have hydroxyl group $( - OH)$availability at the anomeric position of cellulose. Hence, cellulose also does not show mutarotation.
Complete step by step answer:
We must understand that mutarotation is a process that changes the optical rotation of compounds in the aqueous solution. This is because of the change in the equipoise in between two anomers.
Let’s we discuss the structure of these options in detail
We have to remember that the maltose is a reducing sugar which has a hydroxyl group on the ring. It can exhibits mutarotation in aqueous solution, because the α and β-isomers that are formed by the different conformations of the anomeric carbon exhibit different specific rotations, and in aqueous solutions, these two forms are in equilibrium.

And we must know that the disaccharide sugar is lactose which is available in the daily product milk sugar. And it comprises one glucose molecule linked to a galactose molecule by an β(1→4)-glycosidic linkage. Hence, It is a reducing sugar and hence shows mutarotation.

We must understand that the Sucrose does not show mutarotation. However, this property of mutarotation is not exhibited by all sugars. Sucrose does not have free aldehyde (-CHO) or ketone (>C=O) group. Therefore, sucrose is incapable of showing mutarotation.

Glucose is also a reducing sugar show mutarotation.

We can conclude that the Sucrose is not a reducing sugar because it does not have a hydroxyl group in the ring. Hence, sucrose does not show mutarotation.
Note: We can also know that the cellulose also does not show mutarotation as sucrose .This is why because of hemiacetals (or hemiketals). They do not have hydroxyl group $( - OH)$availability at the anomeric position of cellulose. Hence, cellulose also does not show mutarotation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




