
Which of these is a monomer of poly acrylonitrile?
(A) $C{{H}_{2}}O$
(B) $C{{H}_{2}}=CH-CHO$
(C) $C{{H}_{3}}C{{H}_{2}}-N=O$
(D) None of these
Answer
584.1k+ views
Hint: Nitrile is the name given to the CN group. Polymer of polyacrylonitrile is acrylonitrile. It is a free radical vinyl polymerization through which polyacrylonitrile is formed. Acrylonitrile is used as raw material for the manufacture of acrylic and modacrylic fibers.
Complete step by step answer: The monomer of polyacrylonitrile is acrylonitrile and the chemical formula is ${{C}_{3}}{{H}_{3}}N$.
Acrylonitrile is obtained by reacting propylene with ammonia and oxygen in the presence of catalysts. It is a flammable liquid that is highly toxic if ingested and is a known carcinogen.
The structure of acrylonitrile is as follows :
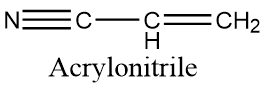
Polyacrylonitrile (PAN), a synthetic resin prepared by the polymerization of acrylonitrile. A member of the important family of acrylic resins, it is a hard,rigid thermoplastic material that is resistant to most solvents and chemicals. Slow to burn and of low permeability to gases. Most polyacrylonitrile is produced as acrylic and modacrylic fibre, a common substitute for wool in clothing and home furnishings.
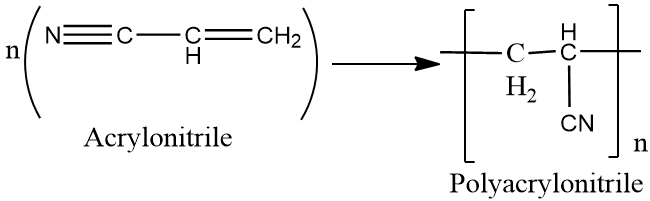
Polyacrylonitrile may cause eye and skin irritation,respiratory and digestive tract irritation. It is metabolized to cyanide in the body, which may cause headache, dizziness, weakness, unconsciousness, convulsions, coma and possible death.
Hence, D is the correct answer.
Note: Acrylonitrile is produced by catalytic ammoxidation of propylene also known as the SOHIO process. In the SOHIO process, propylene, ammonia and air(oxidizer) are passed through a fluidized bed reactor containing the catalyst at $400-{{510}^{{}^\circ }}C$ and 50-200kPa.
Complete step by step answer: The monomer of polyacrylonitrile is acrylonitrile and the chemical formula is ${{C}_{3}}{{H}_{3}}N$.
Acrylonitrile is obtained by reacting propylene with ammonia and oxygen in the presence of catalysts. It is a flammable liquid that is highly toxic if ingested and is a known carcinogen.
The structure of acrylonitrile is as follows :
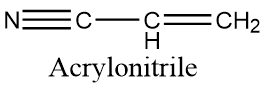
Polyacrylonitrile (PAN), a synthetic resin prepared by the polymerization of acrylonitrile. A member of the important family of acrylic resins, it is a hard,rigid thermoplastic material that is resistant to most solvents and chemicals. Slow to burn and of low permeability to gases. Most polyacrylonitrile is produced as acrylic and modacrylic fibre, a common substitute for wool in clothing and home furnishings.

Polyacrylonitrile may cause eye and skin irritation,respiratory and digestive tract irritation. It is metabolized to cyanide in the body, which may cause headache, dizziness, weakness, unconsciousness, convulsions, coma and possible death.
Hence, D is the correct answer.
Note: Acrylonitrile is produced by catalytic ammoxidation of propylene also known as the SOHIO process. In the SOHIO process, propylene, ammonia and air(oxidizer) are passed through a fluidized bed reactor containing the catalyst at $400-{{510}^{{}^\circ }}C$ and 50-200kPa.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




