
Which one of the following is expected to exhibit resonance?
A. \[C{O_2}\]
B. $N{H_4}^ + $
C. $HCN$
D. $N{O_{_2}}^ - $
Answer
493.8k+ views
Hint: The phenomenon of the resonance was firstly described by Heisenberg to explain resonating properties of the molecule. Resonance is observed in the molecules where one structure failed to explain the properties of the molecule.
Complete answer:
Some basic rules of writing resonating structures are-
the contributing structure of any molecule should have the same position of atoms.
Number of unpaired electrons must be the same in contributing structures.
Energy level of all the contributing structures must be close.
For a molecule of \[C{O_2}\]- total number of valence electrons in the molecule is the sum of valence electrons of carbon and oxygen atoms. Carbon atoms have a total $4$ electron in its outermost shell and oxygen atom has $6$ electrons. Lewis dot structure of the compound is shown as-
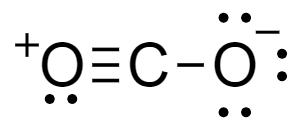
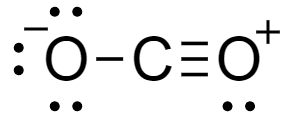
From the structure we see that charge over the molecule is distributed between the two atoms of oxygen separated by carbon. There are two oxygen atoms which contribute to the resonance of the molecule.
For molecules of $N{H_4}^ + $- total number of valence electrons is $5$ electrons of nitrogen and $4$electrons of hydrogen but due to positive charge the total number of electrons becomes $8$. Lewis dot diagram of the molecule is-
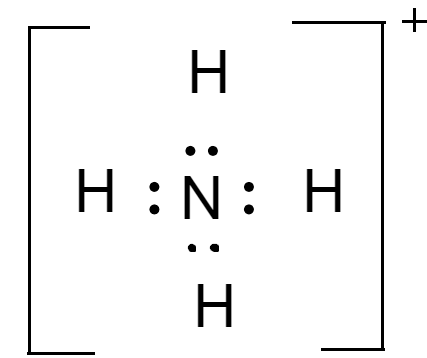
Since, there is no resonating structure possible for this molecule, $N{H_4}^ + $ does not show resonance.
For molecules of $HCN$- total number of electrons are $4$ electrons of carbon, $5$ electrons of nitrogen and $1$ electrons of hydrogen atom. Lewis dot structure of the molecule is shown as-
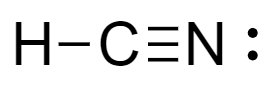
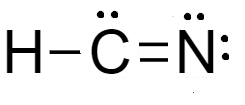
From the structure we see that there is development of partial charge over the nitrogen atom and carbon atom. Therefore, it shows little resonance.
For a molecule of $N{O_{_2}}^ - $- total number of electrons in a molecule are $5$ electrons of nitrogen atom, $12$ electrons from two atoms of oxygen and $1$ electron from the charge. The Lewis structure of the molecule is shown as-
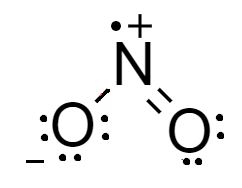
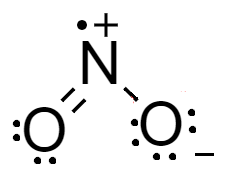
From the Lewis structure we see that charge on the nitrogen atom and oxygen atom are at adjacent locations and there are two oxygen atoms which contribute in resonance. Therefore, it is resonating in nature.
$ \Rightarrow $ Hence, $N{O_{_2}}^ - $ shows resonance. Therefore, option $\left( D \right)$ is the correct option.
Note:
As the number of resonating structures increases, stability of the molecule also increases. Bond length in a molecule becomes equivalent due to resonance. However, the concept of resonance is totally theoretical.
Complete answer:
Some basic rules of writing resonating structures are-
the contributing structure of any molecule should have the same position of atoms.
Number of unpaired electrons must be the same in contributing structures.
Energy level of all the contributing structures must be close.
For a molecule of \[C{O_2}\]- total number of valence electrons in the molecule is the sum of valence electrons of carbon and oxygen atoms. Carbon atoms have a total $4$ electron in its outermost shell and oxygen atom has $6$ electrons. Lewis dot structure of the compound is shown as-
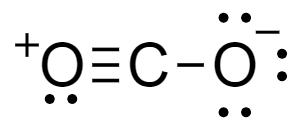
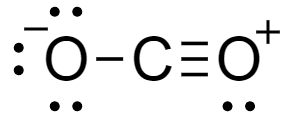
From the structure we see that charge over the molecule is distributed between the two atoms of oxygen separated by carbon. There are two oxygen atoms which contribute to the resonance of the molecule.
For molecules of $N{H_4}^ + $- total number of valence electrons is $5$ electrons of nitrogen and $4$electrons of hydrogen but due to positive charge the total number of electrons becomes $8$. Lewis dot diagram of the molecule is-

Since, there is no resonating structure possible for this molecule, $N{H_4}^ + $ does not show resonance.
For molecules of $HCN$- total number of electrons are $4$ electrons of carbon, $5$ electrons of nitrogen and $1$ electrons of hydrogen atom. Lewis dot structure of the molecule is shown as-
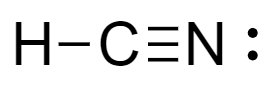
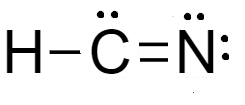
From the structure we see that there is development of partial charge over the nitrogen atom and carbon atom. Therefore, it shows little resonance.
For a molecule of $N{O_{_2}}^ - $- total number of electrons in a molecule are $5$ electrons of nitrogen atom, $12$ electrons from two atoms of oxygen and $1$ electron from the charge. The Lewis structure of the molecule is shown as-


From the Lewis structure we see that charge on the nitrogen atom and oxygen atom are at adjacent locations and there are two oxygen atoms which contribute in resonance. Therefore, it is resonating in nature.
$ \Rightarrow $ Hence, $N{O_{_2}}^ - $ shows resonance. Therefore, option $\left( D \right)$ is the correct option.
Note:
As the number of resonating structures increases, stability of the molecule also increases. Bond length in a molecule becomes equivalent due to resonance. However, the concept of resonance is totally theoretical.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




