
Which one of the following products is formed when calcium salt of adipic acid is heated?
A.
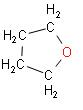
B.
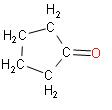
C.
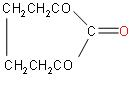
D.
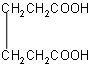



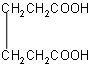
Answer
601.5k+ views
Hint: We need to have a clear idea about the structure of adipic acid. From the reactant and knowing the conditions of the occurrence of the reaction we can predict the product.
Complete step-by-step answer:
To determine the product let us see the reaction first. So, given below is the reaction of adipic acid heating, resulting in the formation of a calcium salt.
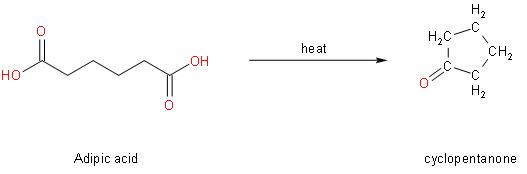
In the above reaction we can see that when adipic acid is heated, the product that is formed is cyclopentanone. It contains one carbon atom less than adipic acid which indicates decarboxylation. Also, a molecule of water is lost which results in cyclization.
So, we can say that the correct answer out of the mentioned options is Option B.
Additional Information:
The reactant that is mentioned in the question is adipic acid. This adipic acid is an important industrial carboxylic acid. It is mainly used in the production of nylon.
The nylon, which has a protein like structure is further processed into fibres for applications in carpenting, automobile tire cord, and clothing. Adipic acid is also used to manufacture plasticizers and lubricant components.
Adipic acid exerts a drying action on the human skin. Ingestion of a large amount of adipic acid causes gastrointestinal irritation.
Note: The product that is formed, cyclopentanone, it is a colourless volatile liquid. This compound has a structure which is similar to the structure of cyclopentane, consisting of a five membered ring containing ketone functional groups.
Complete step-by-step answer:
To determine the product let us see the reaction first. So, given below is the reaction of adipic acid heating, resulting in the formation of a calcium salt.

In the above reaction we can see that when adipic acid is heated, the product that is formed is cyclopentanone. It contains one carbon atom less than adipic acid which indicates decarboxylation. Also, a molecule of water is lost which results in cyclization.
So, we can say that the correct answer out of the mentioned options is Option B.
Additional Information:
The reactant that is mentioned in the question is adipic acid. This adipic acid is an important industrial carboxylic acid. It is mainly used in the production of nylon.
The nylon, which has a protein like structure is further processed into fibres for applications in carpenting, automobile tire cord, and clothing. Adipic acid is also used to manufacture plasticizers and lubricant components.
Adipic acid exerts a drying action on the human skin. Ingestion of a large amount of adipic acid causes gastrointestinal irritation.
Note: The product that is formed, cyclopentanone, it is a colourless volatile liquid. This compound has a structure which is similar to the structure of cyclopentane, consisting of a five membered ring containing ketone functional groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




