
Which one of the following statements about ${C_2}$ molecule is wrong?
A.The bond order of ${C_2}$ is 2.
B.In vapour phase, ${C_2}$ molecule is diamagnetic.
C.Double bond in ${C_2}$ molecule consists of both $\pi $- bonds because of the presence of 4 electrons in two $\pi $ - molecular orbitals.
D.Double bond in ${C_2}$ molecule consists of one $\sigma $ - bond and one $\pi $ - bond.
Answer
589.8k+ views
Hint: ${C_2}$ is a component of vapours of carbon. According to a research paper, carbon vapours contain around 28% ${C_2}$, but this depends on the temperature and pressure.
Complete step by step answer:
The electrons in ${C_2}$ are distributed among the atomic orbitals according to Aufbau principle. This produces unique quantum states, with corresponding energy levels. The quantum state which has the lowest energy level is known as the ground state. The ground state of ${C_2}$ is a singlet state. There are several excited singlet and triplet states that are relatively similar energy to the ground state.
Molecular orbital theory shows that it has two sets of paired electrons in a degenerate $\pi $- bonding set of orbitals. This gives a bond order of 2, which means that there should exist a double bond between the two carbons in a ${C_2}$ .
The electronic configuration of ${C_2}$ is –
\[ \Rightarrow {\left( {\sigma 2s} \right)^2}\;{\text{ }}{({\sigma ^*}2s)^2}\;n{\left( {2px} \right)^2}\;n{\left( {2py} \right)^2}\]
The bond order of ${C_2}$ is –
$\begin{gathered}
\Rightarrow B.O. = \dfrac{{B.E - A.B.E.}}{2} \\
\Rightarrow B.O. = \dfrac{{(8 - 4)}}{2} \\
\Rightarrow B.O. = \dfrac{4}{2} \\
\Rightarrow B.O. = 2 \\
\end{gathered} $
Here, B.O. stands for Bond Order, B.E. stands for Bonding electrons and A.B.E. stands for Anti-bonding electrons.
Here is the molecular orbital diagram of ${C_2}$-
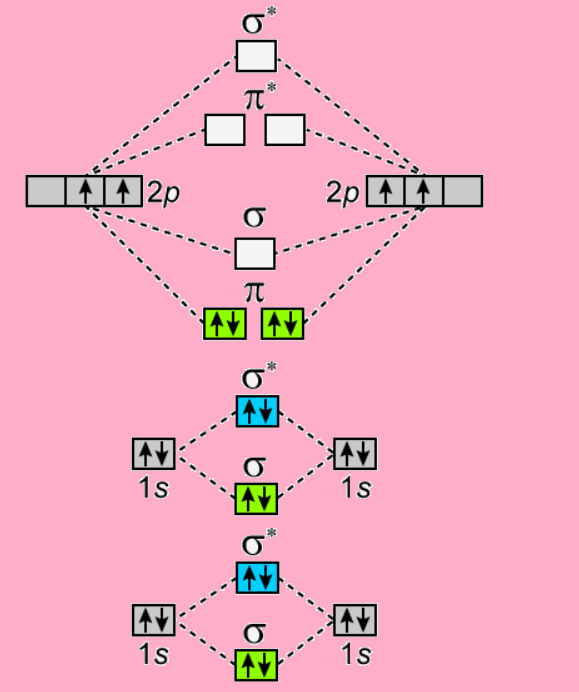
Since the diagram shows two sets of electron pairs in $\pi $orbital, the two bonds are $\pi $- bonds.
Hence, the option (D) is wrong.
Note:
The various quantum states of dicarbon form significant proportions of dicarbon under ambient conditions.
Complete step by step answer:
The electrons in ${C_2}$ are distributed among the atomic orbitals according to Aufbau principle. This produces unique quantum states, with corresponding energy levels. The quantum state which has the lowest energy level is known as the ground state. The ground state of ${C_2}$ is a singlet state. There are several excited singlet and triplet states that are relatively similar energy to the ground state.
Molecular orbital theory shows that it has two sets of paired electrons in a degenerate $\pi $- bonding set of orbitals. This gives a bond order of 2, which means that there should exist a double bond between the two carbons in a ${C_2}$ .
The electronic configuration of ${C_2}$ is –
\[ \Rightarrow {\left( {\sigma 2s} \right)^2}\;{\text{ }}{({\sigma ^*}2s)^2}\;n{\left( {2px} \right)^2}\;n{\left( {2py} \right)^2}\]
The bond order of ${C_2}$ is –
$\begin{gathered}
\Rightarrow B.O. = \dfrac{{B.E - A.B.E.}}{2} \\
\Rightarrow B.O. = \dfrac{{(8 - 4)}}{2} \\
\Rightarrow B.O. = \dfrac{4}{2} \\
\Rightarrow B.O. = 2 \\
\end{gathered} $
Here, B.O. stands for Bond Order, B.E. stands for Bonding electrons and A.B.E. stands for Anti-bonding electrons.
Here is the molecular orbital diagram of ${C_2}$-

Since the diagram shows two sets of electron pairs in $\pi $orbital, the two bonds are $\pi $- bonds.
Hence, the option (D) is wrong.
Note:
The various quantum states of dicarbon form significant proportions of dicarbon under ambient conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




