
Which one of the white phosphorus and red phosphorus is more reactive and why?
Answer
541.8k+ views
Hint: Allotrope defines the term refers to one or more physical forms of a chemical element which occurs in the same physical state. Allotropes show differences in physical and chemical properties of an element. Red and white phosphorus are two allotropic forms of phosphorus.
Complete step-by-step answer: White phosphorus is translucent white waxy solid which is poisonous in nature have the property of chemiluminescence i.e. it glows in dark. It has a discrete tetrahedral like structure which can be shown as:
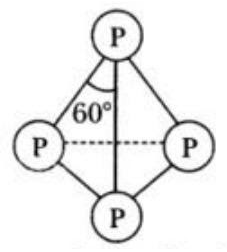
The bond length of phosphorus – phosphorus molecule is 225 pm and the angle between them is 60. Due to angular strain in the molecule in presence of air it readily catches fire.
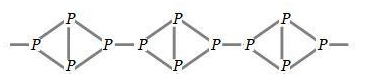
Red phosphorus is odorless, non poisonous in nature and does not glow in the dark. It has iron grey like luster due to which it resembles red color and known as red phosphorus. Red phosphorus is polymeric in nature which consists of chain of ${{P}_{4}}$tetrahedral linked together which can be shown as:
Out of these two allotropes, white phosphorus is less stable than red and therefore white phosphorus is more reactive in nature under normal conditions because of angular strain in ${{P}_{4}}$molecules.
Note:Phosphorus is an important plant nutrient and it is used in fertilizer production by phosphate compounds. Like the cycles of biological carbon and nitrogen there is also an important cycle for phosphorus as well. Red phosphorus is used in making protective matches, pyrotechnics and explosive bombs.
Complete step-by-step answer: White phosphorus is translucent white waxy solid which is poisonous in nature have the property of chemiluminescence i.e. it glows in dark. It has a discrete tetrahedral like structure which can be shown as:

The bond length of phosphorus – phosphorus molecule is 225 pm and the angle between them is 60. Due to angular strain in the molecule in presence of air it readily catches fire.
Red phosphorus is odorless, non poisonous in nature and does not glow in the dark. It has iron grey like luster due to which it resembles red color and known as red phosphorus. Red phosphorus is polymeric in nature which consists of chain of ${{P}_{4}}$tetrahedral linked together which can be shown as:

Out of these two allotropes, white phosphorus is less stable than red and therefore white phosphorus is more reactive in nature under normal conditions because of angular strain in ${{P}_{4}}$molecules.
Note:Phosphorus is an important plant nutrient and it is used in fertilizer production by phosphate compounds. Like the cycles of biological carbon and nitrogen there is also an important cycle for phosphorus as well. Red phosphorus is used in making protective matches, pyrotechnics and explosive bombs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




