
Which one of these is a monomer of Bakelite?
A. Vinyl acetate
B. Tetrafluoroethylene
C. Formaldehyde
D. Propylene
Answer
233.1k+ views
Hint: Polymers are made up of smaller units that are known as monomers. They bind to other monomers to form a repeating chain of molecules known as polymers. Similarly bakelite is a polymer which is made up of a monomer that has a pungent and suffocating odor.
Complete step by step answer:
- A monomer is a molecule that forms the basic unit of polymers. A monomer is a simple molecule that has two or more binding sites.
- The binding sites are used to form covalent linkages with other monomer molecules to form the macromolecule. This process is known as polymerization.
- All simple molecules do not behave as monomers, but only those molecules which have two or more building sites can act as a monomer.
- For example, ammonia, water, ethanol, etc are not monomers, but alkenes, vinyl chloride, adipic acid, phenol, formaldehyde, etc are monomers.
- Bakelite is a polymer that is made up of two monomers: phenol and formaldehyde. This is a thermosetting polymer.
- Bakelite is the common name for the polymer that is made from the polymerization of phenol and formaldehyde. Formaldehyde has a very pungent and a suffocating odor.
- In this, phenol is reacted with formaldehyde. The controlled reaction in the presence of acidic or basic medium results in the formation of ortho and para hydroxymethyl phenols and their derivatives.
- The process involved is shown below.
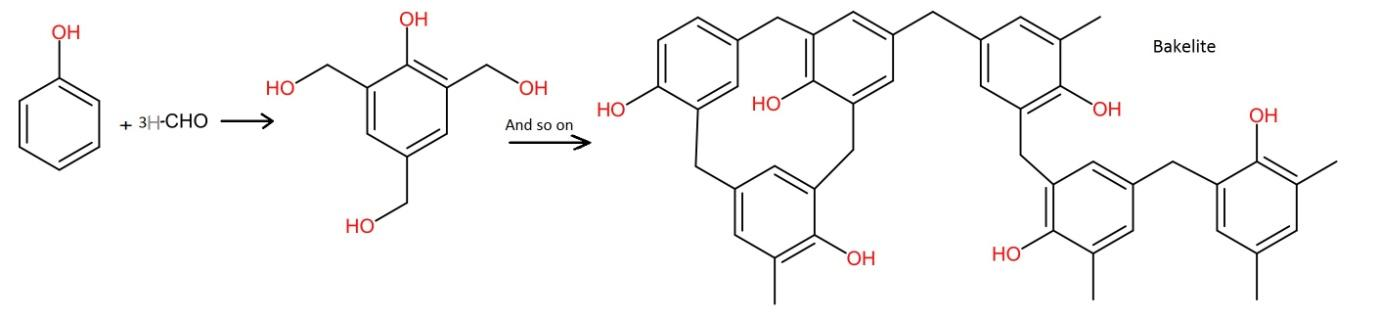
Therefore, formaldehyde and phenol are the monomers of Bakelite. Hence, the correct answer is option (C).
Note: Students tend to confuse between formaldehyde and acetaldehyde. Make sure you do not confuse between the two. Formaldehyde is $HCHO$ and acetaldehyde is $C{H}_{3}CHO$.
Complete step by step answer:
- A monomer is a molecule that forms the basic unit of polymers. A monomer is a simple molecule that has two or more binding sites.
- The binding sites are used to form covalent linkages with other monomer molecules to form the macromolecule. This process is known as polymerization.
- All simple molecules do not behave as monomers, but only those molecules which have two or more building sites can act as a monomer.
- For example, ammonia, water, ethanol, etc are not monomers, but alkenes, vinyl chloride, adipic acid, phenol, formaldehyde, etc are monomers.
- Bakelite is a polymer that is made up of two monomers: phenol and formaldehyde. This is a thermosetting polymer.
- Bakelite is the common name for the polymer that is made from the polymerization of phenol and formaldehyde. Formaldehyde has a very pungent and a suffocating odor.
- In this, phenol is reacted with formaldehyde. The controlled reaction in the presence of acidic or basic medium results in the formation of ortho and para hydroxymethyl phenols and their derivatives.
- The process involved is shown below.

Therefore, formaldehyde and phenol are the monomers of Bakelite. Hence, the correct answer is option (C).
Note: Students tend to confuse between formaldehyde and acetaldehyde. Make sure you do not confuse between the two. Formaldehyde is $HCHO$ and acetaldehyde is $C{H}_{3}CHO$.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




