
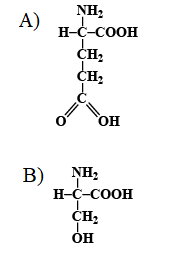
Which one represents the structural formula of basic amino acid?



Answer
523.8k+ views
Hint: Amino acid is made up of two functional groups namely -$N{H_2}$ and $ - COOH.$ The –$COOH$ stands for the carboxylic acid group and the -$N{H_2}$ stands for amino acid group. So, we can say that an amino acid is an organic compound containing one or more amino groups together with one or more carboxylic acid groups in a molecule.
Complete answer:
Amino acid is formed by the molecular arrangement of two or more amino groups with two or more carboxylic acid groups. The amino acids are obtained as the end product of protein hydrolysis. All the amino acids obtained during the process of the protein hydrolysis are alpha amino acids which means that the amino group is attached to the carbon atom that is alpha in respect to the position of carboxylic acid. The amino groups can also be present in the α, β, ¥ position.
Amino acid consists of acidic $ - COOH$ group and basic -$N{H_2}$ group in the same molecule. So, we can say that amino acids exist as Zwitterion in neutral medium. Zwitter ions contain both positive and negative charges in equal numbers that cancel out together and so, they are said to be neutral molecules. Due to the presence of the acidic as well as basic group, α amino acid exists as zwitterion. In the acidic medium, it exists as cation whereas in the basic medium it acts as anion. Every α amino acid has a particular pH at which it exists as a zwitterion.
The pH at which α-amino acid does not migrate under the influence of electric field is called an isoelectric point. The migration of ions under the influence of the electric field is called electrophoresis.
Hence, the correct answer is option (D)
Note: On the basis of the number of carboxylic groups and amino acid groups, the amino acids are classified into different types namely neutral amino acid, acidic amino acid and basic amino acid.
Neutral amino acid contains an equal number of acidic and basic groups. Example is alanine.
Basic amino acid contains more number of amino groups as compared to carboxylic groups. Example is lysine.
The acidic amino acid contains more carboxylic acid than the amino groups. Example is aspartic acid.
Complete answer:
Amino acid is formed by the molecular arrangement of two or more amino groups with two or more carboxylic acid groups. The amino acids are obtained as the end product of protein hydrolysis. All the amino acids obtained during the process of the protein hydrolysis are alpha amino acids which means that the amino group is attached to the carbon atom that is alpha in respect to the position of carboxylic acid. The amino groups can also be present in the α, β, ¥ position.
Amino acid consists of acidic $ - COOH$ group and basic -$N{H_2}$ group in the same molecule. So, we can say that amino acids exist as Zwitterion in neutral medium. Zwitter ions contain both positive and negative charges in equal numbers that cancel out together and so, they are said to be neutral molecules. Due to the presence of the acidic as well as basic group, α amino acid exists as zwitterion. In the acidic medium, it exists as cation whereas in the basic medium it acts as anion. Every α amino acid has a particular pH at which it exists as a zwitterion.
The pH at which α-amino acid does not migrate under the influence of electric field is called an isoelectric point. The migration of ions under the influence of the electric field is called electrophoresis.
Hence, the correct answer is option (D)
Note: On the basis of the number of carboxylic groups and amino acid groups, the amino acids are classified into different types namely neutral amino acid, acidic amino acid and basic amino acid.
Neutral amino acid contains an equal number of acidic and basic groups. Example is alanine.
Basic amino acid contains more number of amino groups as compared to carboxylic groups. Example is lysine.
The acidic amino acid contains more carboxylic acid than the amino groups. Example is aspartic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




