
Which orbital has the highest$\dfrac{n}{k}\gg 1$value?
[A] 7s
[B] 5p
[C] 3d
[D] 4d
Answer
233.1k+ views
Hint: We can solve this question by using the Sommerfeld’s atomic model concept according to which each ‘n’ will have several values of k. We can calculate the $\dfrac{n}{k}$value for each option where ‘n’ will be the energy level and ‘k’ will be the specific s, p, d or f subshell. The highest value of $\dfrac{n}{k}$ will give us the correct answer.
Complete Step by Step Solution:
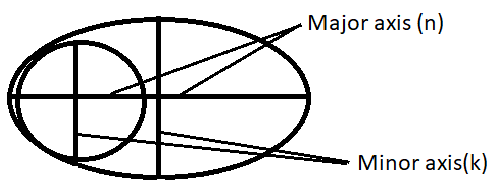
According to Sommerfeld’s atomic model, the orbitals are elliptical and corresponding to each principal quantum number (n) several orbits of varying ellipticity were possible. An ellipse has two axes major and minor and the relation between them was given as-
$\dfrac{n}{k}=\dfrac{major\text{ }axis}{minor\text{ }axis}$
Here, ‘n’ is the principal quantum number. It is the energy level to which the electron belongs and
‘k’ is the azimuthal quantum number which was later changed to ‘l’ for certain mathematical advantages.
Now we will discuss each option to find the orbital with the highest value for$\dfrac{n}{k}\gg 1$,
For s-orbital, $\dfrac{n}{k}=1$as it has a circular orbit therefore, option [A] is incorrect
In option [B], we have 5p orbital,
Here, n will be 5 and corresponding values of k will be-
K = 5 (for s-orbital), 4 (for p-orbital), 3 (for d-orbital), 2(for f-orbital) and 1 (for g-orbital)
Therefore,$\dfrac{n}{k}$for 5p-orbital will be $\dfrac{5}{4}$.
In option [C], we have a 3d orbital.
Here, n will be 3 so we can write the corresponding values of k as-
K = 3 (for s-orbital), 2 (for p-orbital), 1 (for d-orbital)
Therefore,$\dfrac{n}{k}$for 3d-orbital will be $\dfrac{3}{1}$.
In option [D] we have a 4d orbital.
Here, n will be 4 so the corresponding values of k will be –
K= 4 (for s-orbital), 3 (for p-orbital), 2(for d-orbital) and 1 (for f-orbital)
Therefore,$\dfrac{n}{k}$for 4d-orbital will be $\dfrac{4}{2}$.
As we can see from the above calculations that the value of $\dfrac{n}{k}$is highest for a 3d orbital which is 3.
Therefore, the correct answer is option [C] 3d
Note: From the above discussion, we can say that for any given value of ‘n’, k is an integer which is always greater than zero. If the major axis becomes equal to the minor axis, the orbits become circular According to this, we can write that-
If $\dfrac{n}{k}=1$, the orbit is circular and if $\dfrac{n}{k}>1$, the orbit is elliptical.
Complete Step by Step Solution:
According to Sommerfeld’s atomic model, the orbitals are elliptical and corresponding to each principal quantum number (n) several orbits of varying ellipticity were possible. An ellipse has two axes major and minor and the relation between them was given as-
$\dfrac{n}{k}=\dfrac{major\text{ }axis}{minor\text{ }axis}$
Here, ‘n’ is the principal quantum number. It is the energy level to which the electron belongs and
‘k’ is the azimuthal quantum number which was later changed to ‘l’ for certain mathematical advantages.
Now we will discuss each option to find the orbital with the highest value for$\dfrac{n}{k}\gg 1$,
For s-orbital, $\dfrac{n}{k}=1$as it has a circular orbit therefore, option [A] is incorrect
In option [B], we have 5p orbital,
Here, n will be 5 and corresponding values of k will be-
K = 5 (for s-orbital), 4 (for p-orbital), 3 (for d-orbital), 2(for f-orbital) and 1 (for g-orbital)
Therefore,$\dfrac{n}{k}$for 5p-orbital will be $\dfrac{5}{4}$.
In option [C], we have a 3d orbital.
Here, n will be 3 so we can write the corresponding values of k as-
K = 3 (for s-orbital), 2 (for p-orbital), 1 (for d-orbital)
Therefore,$\dfrac{n}{k}$for 3d-orbital will be $\dfrac{3}{1}$.
In option [D] we have a 4d orbital.
Here, n will be 4 so the corresponding values of k will be –
K= 4 (for s-orbital), 3 (for p-orbital), 2(for d-orbital) and 1 (for f-orbital)
Therefore,$\dfrac{n}{k}$for 4d-orbital will be $\dfrac{4}{2}$.
As we can see from the above calculations that the value of $\dfrac{n}{k}$is highest for a 3d orbital which is 3.
Therefore, the correct answer is option [C] 3d
Note: From the above discussion, we can say that for any given value of ‘n’, k is an integer which is always greater than zero. If the major axis becomes equal to the minor axis, the orbits become circular According to this, we can write that-
If $\dfrac{n}{k}=1$, the orbit is circular and if $\dfrac{n}{k}>1$, the orbit is elliptical.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




