
Which oxoacid of sulphur contains S-S bond in its structure?
a.) Disulphurous acid
b.) Disulphuric acid
c.) Perdi Sulphuric acid
d.) Hydrosulfuric acid
Answer
596.1k+ views
Hint: To find the answer to this question, we would have to draw the structure and see. Unfortunately, the only way out to solve these types of questions is to remember how the atoms are bonded in these few acids.
Complete step by step solution:
First let us learn about the oxoacids of sulphur provided to us as options:
Disulphurous acid:
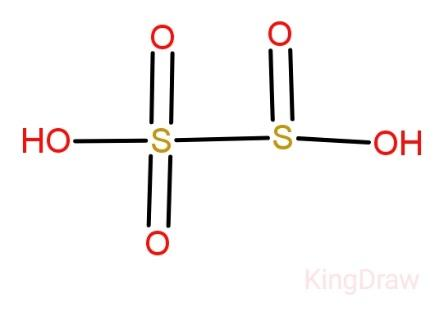
Also known as pyrosulphuric acid, Disulphurous acid cannot exist in free state. The sulphur which is bonded to the three oxygen has an oxidation number of +5 while the other has oxidation number +3. We can see from the structure itself that Disulphurous acid has two directly connected atoms.
Disulphuric acid:
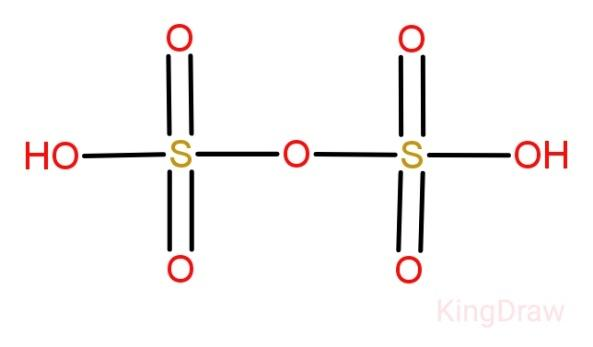
Commonly known as oleum, disulphuric acid is the sulphuric acid analog of an acid anhydride. It is a viscous oily hygroscopic liquid, brown in colour and accompanied by a characteristic odour.
Perdi Sulphuric acid:
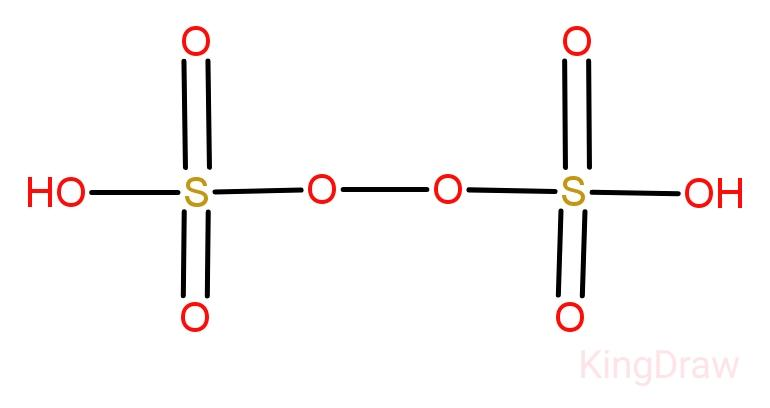
Also known as Marshall’s acid, Perdisulphuric acid is a hygroscopic crystalline solid. Due to its oxidizing and bleaching action it is used in the textile and dyeing industries, and for bleaching soap. It is also used for deodorizing whale and fish oils and animal fats in order to render them suitable for soap making.
Hydrosulfuric acid:
It is an unstable acid with no fixed structure and spontaneously decomposes into sulfur dioxide gas.
Hence, the correct answer is Option (A) Disulphurous acid.
Note:
We must remember that oxoacids of sulphur are chemical compounds containing sulphur, oxygen and hydrogen only. Most of the acids are only important for the salts obtained from them; they do not have any other application. The most popular of these is sulphuric acid with numerous industrial applications.
Complete step by step solution:
First let us learn about the oxoacids of sulphur provided to us as options:
Disulphurous acid:

Also known as pyrosulphuric acid, Disulphurous acid cannot exist in free state. The sulphur which is bonded to the three oxygen has an oxidation number of +5 while the other has oxidation number +3. We can see from the structure itself that Disulphurous acid has two directly connected atoms.
Disulphuric acid:

Commonly known as oleum, disulphuric acid is the sulphuric acid analog of an acid anhydride. It is a viscous oily hygroscopic liquid, brown in colour and accompanied by a characteristic odour.
Perdi Sulphuric acid:

Also known as Marshall’s acid, Perdisulphuric acid is a hygroscopic crystalline solid. Due to its oxidizing and bleaching action it is used in the textile and dyeing industries, and for bleaching soap. It is also used for deodorizing whale and fish oils and animal fats in order to render them suitable for soap making.
Hydrosulfuric acid:
It is an unstable acid with no fixed structure and spontaneously decomposes into sulfur dioxide gas.
Hence, the correct answer is Option (A) Disulphurous acid.
Note:
We must remember that oxoacids of sulphur are chemical compounds containing sulphur, oxygen and hydrogen only. Most of the acids are only important for the salts obtained from them; they do not have any other application. The most popular of these is sulphuric acid with numerous industrial applications.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




