
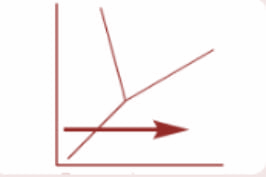
Which process is represented by the arrow on the following phase diagram?
A.Evaporation
B.Deposition
C.Condensation
D.Freezing
E.Sublimation

Answer
578.4k+ views
Hint: There are certain phenomena such as evaporation, sublimation which are performed for separating one substance from another or they are referred to as phase transitions. So, to solve this question we need to know the basic properties of all the phenomena in our daily life.
Complete step by step answer:
Now, let’s discuss all the processes and then determine the correct answer.
1.Evaporation-It is defined as the process by which a liquid or solid is transformed into vapor. When a liquid material becomes a gas, then evaporation occurs. Further, the molecules move and vibrate so rapidly that they disperse as water vapor molecules into the atmosphere.
2.Condensation- This process is just opposite of evaporation. In this process, a gas turns into a liquid and heat energy is lost. For example, water droplets on the surface of glass full of ice-cold water. So, when the water vapor formed from evaporation comes in contact with the cold tumbler, then it loses energy and gets converted to water.
3.Deposition- It is basically a phase transition in which gas transforms into solid without passing through the liquid phase. It is a thermodynamic process. For example, in sub-freezing air, water vapor changes directly to ice without first becoming a liquid.
4.Sublimation- It is the passage or transformation or conversion that substances undergo when passing from one state to another. A substance transforms from a solid to gas by sublimation without even going through a liquid phase. For example, dry ice which is a frozen form of carbon dioxide.
5.Freezing- It is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point.
So, the process represented by the arrow on the phase diagram is a sublimation process as we can see that the physical state changes directly from solid state to gaseous state without entering the liquid state.
Hence, option E is correct.
Note: A range of solids, including water, iodine, solid carbon dioxide i.e. dry ice can sublimate at normal temperatures and pressures. Moreover, other materials can sometimes be made to sublimate by creating conditions of low pressure.
Complete step by step answer:
Now, let’s discuss all the processes and then determine the correct answer.
1.Evaporation-It is defined as the process by which a liquid or solid is transformed into vapor. When a liquid material becomes a gas, then evaporation occurs. Further, the molecules move and vibrate so rapidly that they disperse as water vapor molecules into the atmosphere.
2.Condensation- This process is just opposite of evaporation. In this process, a gas turns into a liquid and heat energy is lost. For example, water droplets on the surface of glass full of ice-cold water. So, when the water vapor formed from evaporation comes in contact with the cold tumbler, then it loses energy and gets converted to water.
3.Deposition- It is basically a phase transition in which gas transforms into solid without passing through the liquid phase. It is a thermodynamic process. For example, in sub-freezing air, water vapor changes directly to ice without first becoming a liquid.
4.Sublimation- It is the passage or transformation or conversion that substances undergo when passing from one state to another. A substance transforms from a solid to gas by sublimation without even going through a liquid phase. For example, dry ice which is a frozen form of carbon dioxide.
5.Freezing- It is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point.
So, the process represented by the arrow on the phase diagram is a sublimation process as we can see that the physical state changes directly from solid state to gaseous state without entering the liquid state.
Hence, option E is correct.
Note: A range of solids, including water, iodine, solid carbon dioxide i.e. dry ice can sublimate at normal temperatures and pressures. Moreover, other materials can sometimes be made to sublimate by creating conditions of low pressure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




