
Which statement is incorrect?
A. pH scale was presented by S.P.L Sorensen
B. pH scale ranges between 0 and 14
C. pH scale is applicable to only non-aqueous solutions.
D. pH scale is applicable to only aqueous solutions
Answer
572.7k+ views
Hint: When measuring pH, the negative logarithm of the concentration of hydrogen is taken. It can be only done if the hydrogen ions are able to move about freely in the solution, it only happens in a specific type of solution.
Complete step by step answer:
In chemistry, pH is referred to as the potential of hydrogen. pH is a standard scale that is used to measure how much acidic or basic a solution is. pH was invented by S.P.L Sorensen and the scale ranges from the value of 0 to 14. As we progress right in the scale, the acidic character reduces and the basic character increases. Solutions which have pH close less than 7 are said to be acidic, whereas those with pH more than 7 are basic.
At pH=7 the solution is said to be neutral, as it has equal amounts of ${{H}^{+}}$ and $O{{H}^{-}}$ ions.
Here is the pH scale:
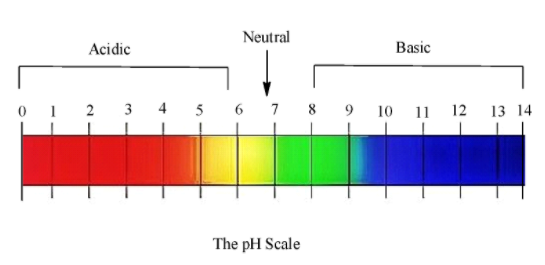
So, on dipping a pH paper in a solution, we get a colour and by identifying the colour, we can get the respective pH values.
Mathematically pH is represented by: $pH=-\log [{{H}^{+}}]$, so pH depends on the concentration of hydrogen ions present in the solution. However, for that to happen the solution must be aqueous. Only in aqueous solutions the ions get dissociated, and dissociation does not take place in non-aqueous solutions.
So, pH scale does not apply only to non-aqueous solutions. Hence, it is the incorrect statement and we obtain our answer as option C.
Note: As $pH=-\log [{{H}^{+}}]$, hence it is a decreasing function. A solution that has more ${{H}^{+}}$ in it will have more pH. Moreover, the pH of pure water at ${{25}^{0}}C$is equal to 7. Temperature also plays a role in determining the pH.
Complete step by step answer:
In chemistry, pH is referred to as the potential of hydrogen. pH is a standard scale that is used to measure how much acidic or basic a solution is. pH was invented by S.P.L Sorensen and the scale ranges from the value of 0 to 14. As we progress right in the scale, the acidic character reduces and the basic character increases. Solutions which have pH close less than 7 are said to be acidic, whereas those with pH more than 7 are basic.
At pH=7 the solution is said to be neutral, as it has equal amounts of ${{H}^{+}}$ and $O{{H}^{-}}$ ions.
Here is the pH scale:

So, on dipping a pH paper in a solution, we get a colour and by identifying the colour, we can get the respective pH values.
Mathematically pH is represented by: $pH=-\log [{{H}^{+}}]$, so pH depends on the concentration of hydrogen ions present in the solution. However, for that to happen the solution must be aqueous. Only in aqueous solutions the ions get dissociated, and dissociation does not take place in non-aqueous solutions.
So, pH scale does not apply only to non-aqueous solutions. Hence, it is the incorrect statement and we obtain our answer as option C.
Note: As $pH=-\log [{{H}^{+}}]$, hence it is a decreasing function. A solution that has more ${{H}^{+}}$ in it will have more pH. Moreover, the pH of pure water at ${{25}^{0}}C$is equal to 7. Temperature also plays a role in determining the pH.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




