
Which statement is/are correct?
\[\begin{align}
& (i)-In\text{ oxymercuration and demercuration reaction, the electrophile is AcO}{{\text{H}}^{-}} \\
& (ii)-In\text{ this reaction, H}{{\text{g}}^{+}}is\text{ reduced to H}{{\text{g}}^{0}} \\
& (iii)-In\text{ this reaction, H}{{\text{g}}^{+2}}\text{ is reduced to H}{{\text{g}}^{+}} \\
& (iv)\text{ - In this reaction, H}{{\text{g}}^{+2}}is\text{ reduced to H}{{\text{g}}^{0}} \\
\end{align}\]
(A)-(i) and (iv)
(B)-(i) and (ii)
(C)-(i) and (iii)
(D)-(iv)
Answer
587.4k+ views
Hint: oxymercuration is first step and demercuration is second step. Mercuric acetate is added as reagent.Hydroxyl group is added across an alkene according to markovnikov rule. Oxymercuration is the addition of oxygen and mercury. Demercuration is removal of mercury.
Complete step by step answer:
-Mercuric acetate acts as reagent Mercuric acetate $Hg{{(OAc)}_{2}}$ is in equilibrium with mercuric acetate ion$H{{g}^{+}}OAc$$H{{g}^{+}}OAc$. Mercuric acetate ion attacks $\pi $ bond of alkene. $AcO{{H}^{-}}$acts as electrophile
An electrophile is an electron deficient molecule.
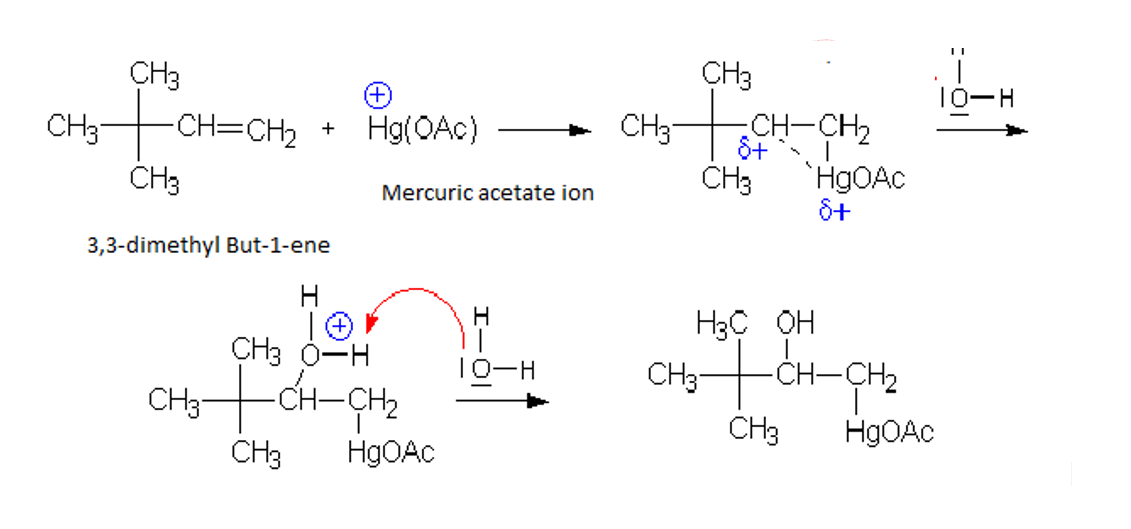 Three membered ring of mercurinium ion is formed, which gets opened by attack of water
Three membered ring of mercurinium ion is formed, which gets opened by attack of water
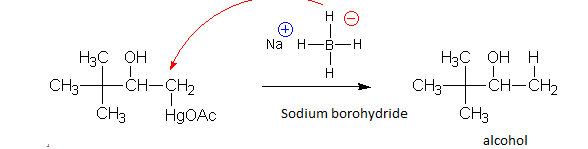
Oxymercuration is followed by reductive demercuration. It is treated with sodium borohydride. Sodium borohydride acts as a reducing agent, so Mercury acetoxy group is replaced with hydrogen and elemental mercury is formed.
In mercury acetate, mercury is present as $H{{g}^{+2}}$ and oxymercuration followed by demercuration results in formation of elemental mercury.
So, In oxymercuration and Demercuration , $AcO{{H}^{-}}$ acts as electrophile and $H{{g}^{+2}}$ is reduced to Hg.
The addition of $H{{g}^{+}}OAc$ takes place according to markovnikov rule.
Markovnikov rule states, In an unsymmetrical alkene, negative part of reagent i.e. $O{{H}^{-}}$ is added to a carbon atom having less number of hydrogen atoms across double bonds.
Markovnikov rule is used to determine major products.
Note: alkenes do not undergo hydration reaction easily under mild conditions, carbocation rearrangement can also be used but it gives low yield. Oxymercuration and demercuration is faster and yield is high. Also, this method is useful because strong acids are not used.
Complete step by step answer:
-Mercuric acetate acts as reagent Mercuric acetate $Hg{{(OAc)}_{2}}$ is in equilibrium with mercuric acetate ion$H{{g}^{+}}OAc$$H{{g}^{+}}OAc$. Mercuric acetate ion attacks $\pi $ bond of alkene. $AcO{{H}^{-}}$acts as electrophile
An electrophile is an electron deficient molecule.


Oxymercuration is followed by reductive demercuration. It is treated with sodium borohydride. Sodium borohydride acts as a reducing agent, so Mercury acetoxy group is replaced with hydrogen and elemental mercury is formed.
In mercury acetate, mercury is present as $H{{g}^{+2}}$ and oxymercuration followed by demercuration results in formation of elemental mercury.
So, In oxymercuration and Demercuration , $AcO{{H}^{-}}$ acts as electrophile and $H{{g}^{+2}}$ is reduced to Hg.
The addition of $H{{g}^{+}}OAc$ takes place according to markovnikov rule.
Markovnikov rule states, In an unsymmetrical alkene, negative part of reagent i.e. $O{{H}^{-}}$ is added to a carbon atom having less number of hydrogen atoms across double bonds.
Markovnikov rule is used to determine major products.
Note: alkenes do not undergo hydration reaction easily under mild conditions, carbocation rearrangement can also be used but it gives low yield. Oxymercuration and demercuration is faster and yield is high. Also, this method is useful because strong acids are not used.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




