
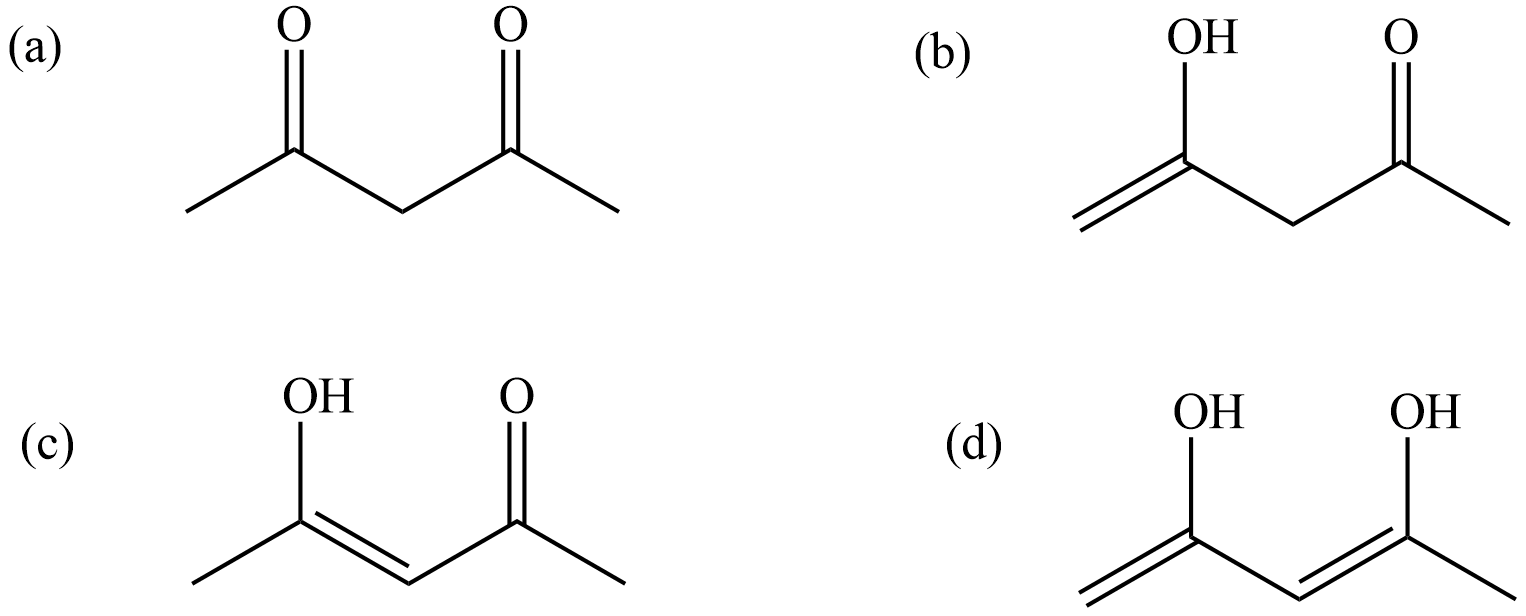
Which structure is most stable?

Answer
577.5k+ views
Hint:. The given structures are the resonance forms of the organic compound acetylacetone. The stability of this compound is found from understanding the keto form and enol forms of isomeric structures it exhibits.
Complete step by step answer:
The structure (a) is the keto form of acetylacetone in which there are two keto groups. It is named in IUPAC norms as 2, 4-pentanedione. This keto form undergoes intramolecular rearrangement to form another structure which is known as enol from.
The structure shown in (b) and (c) are the enol forms of the acetyl acetone. It is a resonance structure and is interconvertible with 2, 4-pentanedione. As keto form and enol forms are both interconvertible they are called tautomers and this phenomenon is known as Keto-enol tautomerism. When the enol form is observed the lone pair of electrons on the oxygen atom participates in delocalization between all the carbons in the chain thus it becomes resonance stabilized while in keto form the compound is destabilized by the two electronegative oxygen atoms which try to attract the electron cloud towards themselves as they are electron withdrawing nature. But the structure (d) is also an enol form of acetylacetone in which there occurs intra molecular hydrogen bonding between the oxygen atom of one OH and the hydrogen atom of the other. This stabilizes the compound to a large extent by the chelation due to hydrogen bonding. So, the correct answer is “Option D”.
Note: In the structure shown in option (d), there are two hydroxyl groups and two double bonds (diene).
More is the enol percentage; more will be its stability, this way also the structure can be considered as more stable.
Complete step by step answer:
The structure (a) is the keto form of acetylacetone in which there are two keto groups. It is named in IUPAC norms as 2, 4-pentanedione. This keto form undergoes intramolecular rearrangement to form another structure which is known as enol from.
The structure shown in (b) and (c) are the enol forms of the acetyl acetone. It is a resonance structure and is interconvertible with 2, 4-pentanedione. As keto form and enol forms are both interconvertible they are called tautomers and this phenomenon is known as Keto-enol tautomerism. When the enol form is observed the lone pair of electrons on the oxygen atom participates in delocalization between all the carbons in the chain thus it becomes resonance stabilized while in keto form the compound is destabilized by the two electronegative oxygen atoms which try to attract the electron cloud towards themselves as they are electron withdrawing nature. But the structure (d) is also an enol form of acetylacetone in which there occurs intra molecular hydrogen bonding between the oxygen atom of one OH and the hydrogen atom of the other. This stabilizes the compound to a large extent by the chelation due to hydrogen bonding. So, the correct answer is “Option D”.
Note: In the structure shown in option (d), there are two hydroxyl groups and two double bonds (diene).
More is the enol percentage; more will be its stability, this way also the structure can be considered as more stable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




