
Which type of isomerism is shown in$2,3 - {\text{dichlorobutane}}$ ?
A) Structural B) Geometric C) Optical D) Diastereo
Answer
537.6k+ views
Hint :-In $2,3 - {\text{dichlorobutane}}$, the carbon on ${2^{{\text{nd}}}}$ and ${3^{{\text{rd}}}}$ position are chiral carbons(the carbon atom containing four different groups as its substituents).When we draw the structures of isomers, we also see that the images are mirror images of each other but they are not super imposable.
Complete Step By Step Answer:
$2,3 - {\text{dichlorobutane}}$ shows optical isomerism as it fulfills the following conditions-
1. The compound should have chiral carbons.
Ex-It should contain different atoms as its substituent like

2. The isomers must have non-super imposable mirror images.
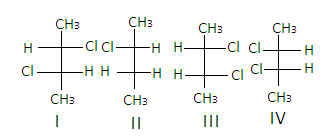
It contains chiral carbons in ${2^{{\text{nd}}}}$ and${3^{{\text{rd}}}}$ positions as all the four groups attached to the carbons are different and it forms $4$ optical isomers which are non -super imposable mirror images of each other.
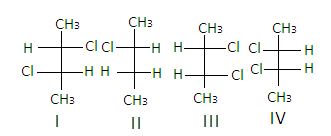
Hence, the correct answer is ‘C’.
Additional Information:
Let us also understand about other types of isomerism-
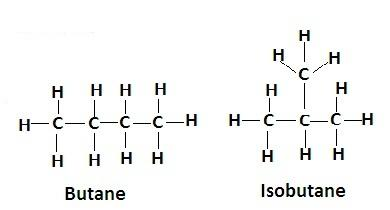
1. Structural isomerism is the isomerism in which the molecular formula is the same but the structure is different as the atoms have different arrangements. Ex- butane and isobutane have same molecular formula but their structures are different-
2. Geometrical isomerism is the isomerism in which spatial arrangement of ligands is changed. When the identical ligands occupy adjacent position it is called cis-isomer and when they are opposite to one other, it is called Trans isomer. It is also called cis-tans isomerism. Ex-
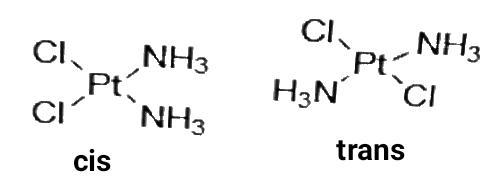
3. Diastereo isomerism is like optical isomerism but the isomers are not mirror images in this isomerism.
In the structure of$2,3 - {\text{dichlorobutane}}$, the structures I and III are diastereomers.
Note :
Optical isomers are the compounds having the same molecular formula but different spatial arrangement of atoms .The isomers form non-super imposable mirror images called enantiomers.
The mirror images when put upon each other are not superimposed because the arrangement of atoms differs.
Complete Step By Step Answer:
$2,3 - {\text{dichlorobutane}}$ shows optical isomerism as it fulfills the following conditions-
1. The compound should have chiral carbons.
Ex-It should contain different atoms as its substituent like

2. The isomers must have non-super imposable mirror images.
It contains chiral carbons in ${2^{{\text{nd}}}}$ and${3^{{\text{rd}}}}$ positions as all the four groups attached to the carbons are different and it forms $4$ optical isomers which are non -super imposable mirror images of each other.

Hence, the correct answer is ‘C’.
Additional Information:
Let us also understand about other types of isomerism-
1. Structural isomerism is the isomerism in which the molecular formula is the same but the structure is different as the atoms have different arrangements. Ex- butane and isobutane have same molecular formula but their structures are different-

2. Geometrical isomerism is the isomerism in which spatial arrangement of ligands is changed. When the identical ligands occupy adjacent position it is called cis-isomer and when they are opposite to one other, it is called Trans isomer. It is also called cis-tans isomerism. Ex-
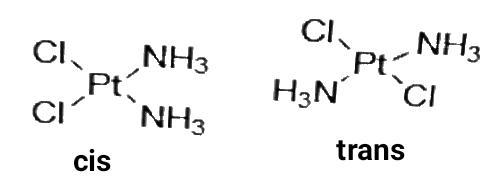
3. Diastereo isomerism is like optical isomerism but the isomers are not mirror images in this isomerism.
In the structure of$2,3 - {\text{dichlorobutane}}$, the structures I and III are diastereomers.

Note :
Optical isomers are the compounds having the same molecular formula but different spatial arrangement of atoms .The isomers form non-super imposable mirror images called enantiomers.
The mirror images when put upon each other are not superimposed because the arrangement of atoms differs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




