
White phosphorous (${P_4}$) has:
A. four $P - P$ single bonds
B. four lone pair of electrons
C. $P - P - P$ angle of ${60^\circ}$
D. six $P - P$ single bonds
Answer
531.4k+ views
Hint: Four phosphorus atoms in white phosphorus (${P_4}$) molecule arranged in tetrahedral structure and form ring structure. White phosphorus is less stable and therefore, more reactive than the other solid phases under normal conditions because of angular strain in the ${P_4}$ molecule. It readily catches the fire in the air.
Complete answer:
White phosphorous (${P_4}$) molecule has tetrahedral geometry. Electronic configuration of phosphorus atom: $1{s^2}{\text{ 2}}{s^2}{\text{ 2}}{{\text{p}}^6}{\text{ 3}}{s^2}{\text{ 3p}}_x^1{\text{ 3p}}_y^1{\text{ 3p}}_z^1$ . Phosphorus atom has 5 valence electrons. Valency of phosphorus atom is 5 and each phosphorus atom forms 3 bonds in a (${P_4}$) molecule with other phosphorus atoms and one lone pair of electrons present on each phosphorus atom. Structure of white phosphorous (${P_4}$) molecules as:
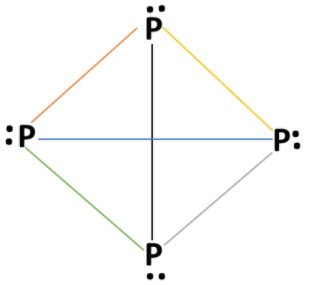
Each P atom has one lone pair of electrons and each covalent bond has two electrons of a bond pair. In a white phosphorus molecule, there are four lone pairs of electrons on four phosphorus atoms and six $P - P$ single bonds are present. $P - P - P$ bond angle is ${60^\circ}$ in white phosphorus molecule.
Hence, correct answers are (B) and (C).
Additional Information: Tetrahedral arrangement of white phosphorus molecule results in ring strain and instability. White phosphorus (${P_4}$) molecule is not a planar molecule, it is a 3D molecule. White phosphorus is a translucent white waxy solid. It is insoluble in water and soluble in carbon disulphide.
Note: Valency of phosphorus atom is 5 and total four phosphorus atoms are present in a white phosphorus molecule. Total valence electrons in white phosphorus molecules will be $5{\text{ x 4 = 20}}$ and lone pairs of electrons are 4 (each phosphorus atom has one lone pair of electrons).
Complete answer:
White phosphorous (${P_4}$) molecule has tetrahedral geometry. Electronic configuration of phosphorus atom: $1{s^2}{\text{ 2}}{s^2}{\text{ 2}}{{\text{p}}^6}{\text{ 3}}{s^2}{\text{ 3p}}_x^1{\text{ 3p}}_y^1{\text{ 3p}}_z^1$ . Phosphorus atom has 5 valence electrons. Valency of phosphorus atom is 5 and each phosphorus atom forms 3 bonds in a (${P_4}$) molecule with other phosphorus atoms and one lone pair of electrons present on each phosphorus atom. Structure of white phosphorous (${P_4}$) molecules as:

Each P atom has one lone pair of electrons and each covalent bond has two electrons of a bond pair. In a white phosphorus molecule, there are four lone pairs of electrons on four phosphorus atoms and six $P - P$ single bonds are present. $P - P - P$ bond angle is ${60^\circ}$ in white phosphorus molecule.
Hence, correct answers are (B) and (C).
Additional Information: Tetrahedral arrangement of white phosphorus molecule results in ring strain and instability. White phosphorus (${P_4}$) molecule is not a planar molecule, it is a 3D molecule. White phosphorus is a translucent white waxy solid. It is insoluble in water and soluble in carbon disulphide.
Note: Valency of phosphorus atom is 5 and total four phosphorus atoms are present in a white phosphorus molecule. Total valence electrons in white phosphorus molecules will be $5{\text{ x 4 = 20}}$ and lone pairs of electrons are 4 (each phosphorus atom has one lone pair of electrons).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




