
Why is $AlC{{l}_{3}}$ a good catalyst?
Answer
548.1k+ views
Hint: A good catalyst is one which has the ability to lend and give electrons or ions during a chemical reaction. $AlC{{l}_{3}}$ has vacant d-orbitals which enables it to attract electron pairs from others.
Complete answer:
Aluminium chloride is a powerful Lewis acid. Lewis acid is one which has the ability to accept a pair of electrons. We use aluminium chloride mostly in the chlorination process in the reactions like Friedel Crafts. The organizing and breaking of these bonds which results in halogenation are done by using aluminium chloride.
The chlorination of aromatic molecules like benzene is facilitated by aluminium chloride. The regeneration of $AlC{{l}_{3}}$ takes place in this process and HCl is produced as a by-product. The Friedel Crafts reaction also uses $AlC{{l}_{3}}$ as a catalyst and aromatic ketones are produced along with HCl as a by-product.
Aluminium has 6 electrons in its outermost shell in $AlC{{l}_{3}}$ and for this reason it is electron deficient. Also, there is a vacant d-orbital in aluminium. Thus, it has the ability to accept a pair of electrons. This makes $AlC{{l}_{3}}$ a great catalyst in different reactions which requires the donation of electrons.
The chlorination process of benzene using $AlC{{l}_{3}}$ is given below.
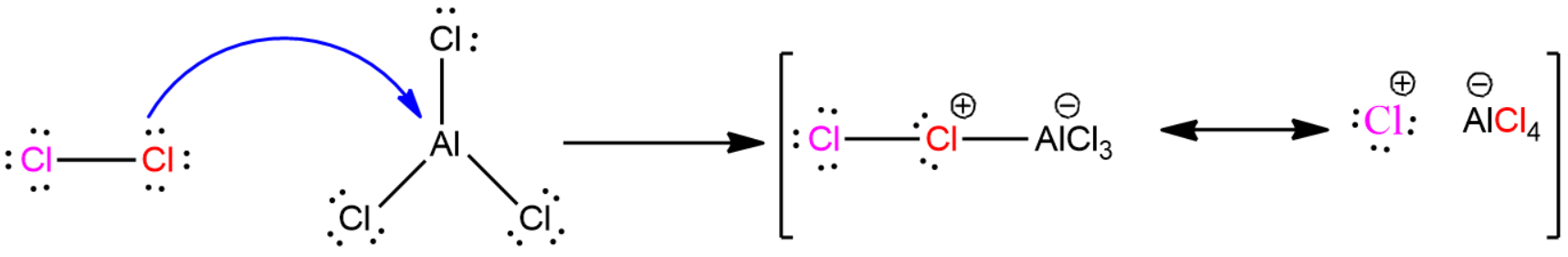
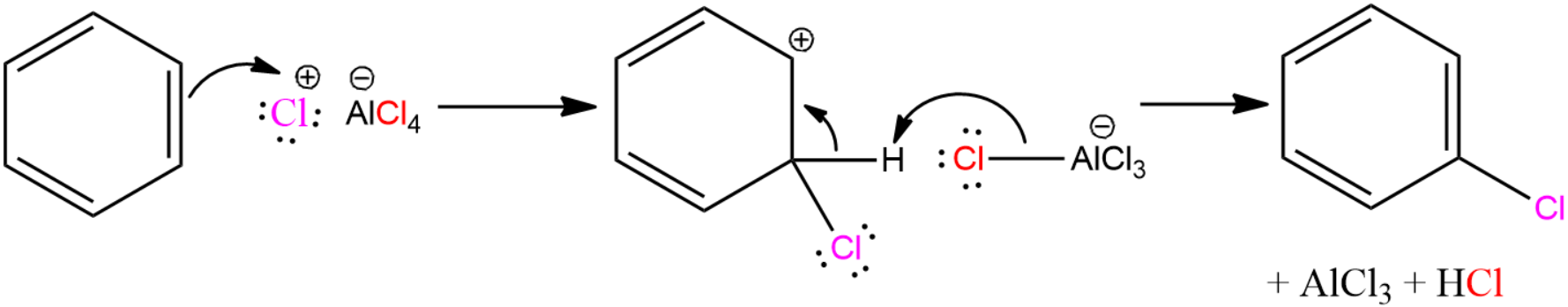
This is the reason why aluminium chloride is considered to be a good catalyst.
Note:
$AlC{{l}_{3}}$ is a lewis acid not a lewis base. A lewis base can donate a pair of electrons but a lewis base will accept a pair of electrons. Don’t confuse between them.
Complete answer:
Aluminium chloride is a powerful Lewis acid. Lewis acid is one which has the ability to accept a pair of electrons. We use aluminium chloride mostly in the chlorination process in the reactions like Friedel Crafts. The organizing and breaking of these bonds which results in halogenation are done by using aluminium chloride.
The chlorination of aromatic molecules like benzene is facilitated by aluminium chloride. The regeneration of $AlC{{l}_{3}}$ takes place in this process and HCl is produced as a by-product. The Friedel Crafts reaction also uses $AlC{{l}_{3}}$ as a catalyst and aromatic ketones are produced along with HCl as a by-product.
Aluminium has 6 electrons in its outermost shell in $AlC{{l}_{3}}$ and for this reason it is electron deficient. Also, there is a vacant d-orbital in aluminium. Thus, it has the ability to accept a pair of electrons. This makes $AlC{{l}_{3}}$ a great catalyst in different reactions which requires the donation of electrons.
The chlorination process of benzene using $AlC{{l}_{3}}$ is given below.


This is the reason why aluminium chloride is considered to be a good catalyst.
Note:
$AlC{{l}_{3}}$ is a lewis acid not a lewis base. A lewis base can donate a pair of electrons but a lewis base will accept a pair of electrons. Don’t confuse between them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




