
With reference to the diagram given, the Van der Waals radius is equal to:
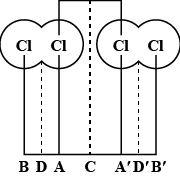
(A) A – A
(B) B – A
(C) B – D
(D) A – C

Answer
568.5k+ views
Hint: Van der Waals radius of any atom is the radius representing the distance of an imaginary sphere to another sphere. The value of Van der Waals radius is measured in picometers.
Complete Solution :
Let us have a quick look towards some facts before answering the actual question;
To describe the size and the distance between the atoms we have three approaches i.e. covalent bond distance, covalent radius and Van der Waals radius. Focussing on Van der Waals radius; it is the half of the distance between the closest approach of two non-bonded atoms of a given substance.
- In short, the Van der Waals radius is one half of the distance between the nuclei of the two non-bonded adjacent atoms which belongs to the two neighbouring molecules of an element in the solid state.
Hence, from the above given diagram and also the mentioned definitions we can say that the Van der Waals radius will be equal to A – C.
So, the correct answer is “Option D”.
Note: Do note that the option (A) can never be the answer as, it does not show or specify any distance.
Also, as mentioned above, covalent bond distance is the distance between the nuclei of the two bonded atoms and covalent radius is the half of the internuclear separation between the nuclei of the two single bonded atoms.
Complete Solution :
Let us have a quick look towards some facts before answering the actual question;
To describe the size and the distance between the atoms we have three approaches i.e. covalent bond distance, covalent radius and Van der Waals radius. Focussing on Van der Waals radius; it is the half of the distance between the closest approach of two non-bonded atoms of a given substance.
- In short, the Van der Waals radius is one half of the distance between the nuclei of the two non-bonded adjacent atoms which belongs to the two neighbouring molecules of an element in the solid state.
Hence, from the above given diagram and also the mentioned definitions we can say that the Van der Waals radius will be equal to A – C.
So, the correct answer is “Option D”.
Note: Do note that the option (A) can never be the answer as, it does not show or specify any distance.
Also, as mentioned above, covalent bond distance is the distance between the nuclei of the two bonded atoms and covalent radius is the half of the internuclear separation between the nuclei of the two single bonded atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




