
With reference to the first row transition series:
i) Name the metal which possesses maximum number of oxidation states.
ii) Among $Z{{n}^{+2}}$and $C{{u}^{+2}}$ which is colourless?
iii) Between $T{{i}^{+2}}$ and ${{V}^{+2}}$which ion contains more number of unpaired electrons?
Answer
566.4k+ views
Hint: To answer this question we should be aware of the elements that belong to first row transition series and their atomic number which helps us to figure out whether the element has unpaired electrons or not , paramagnetic or diamagnetic etc.
Complete answer:
The last electron in the atoms of these elements enters the d-sub shell belonging to the penultimate shell and are called transition metals.
The elements of first transition series are:
\[S{{c}_{21}},T{{i}_{22}},{{V}_{23}},C{{r}_{24}},M{{n}_{25}},F{{e}_{26}},C{{o}_{27}},N{{i}_{28}},C{{u}_{29}},Z{{n}_{30}}\]
i) Mn (+2,+3,+4,+5,+6,+7) possesses the highest number of oxidation states. This is because the electronic configuration of manganese is $[Ar]3{{d}^{5}}4{{s}^{2}}$and it possesses maximum number of electrons to share as well as lose.
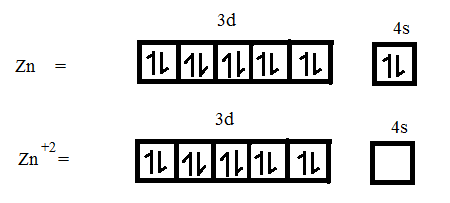
(ii) Atomic number of Zn = 30
Electronic configuration is $[Ar]3{{d}^{10}}4{{s}^{2}}$.
It loses 2 electrons to form $Z{{n}^{+2}}$.
Atomic number of Cu = 29
Electronic configuration is $[Ar]3{{d}^{10}}$
It loses 2 electrons to form $C{{u}^{+2}}$.
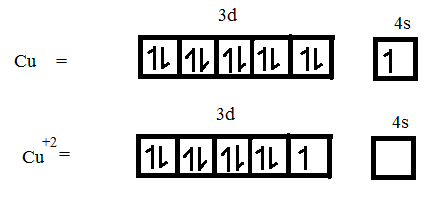
$Z{{n}^{+2}}$ is diamagnetic nature and $C{{u}^{+2}}$ is paramagnetic nature. Since $Z{{n}^{+2}}$ is diamagnetic it is colourless. $C{{u}^{+2}}$ being paramagnetic in nature which has a unpaired electrons that absorbs radiation of certain wavelength and emits back light that imparts some colour.
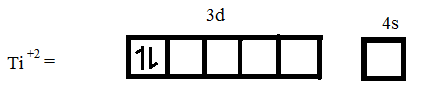
(iii) Atomic number of Ti = 22
Electronic configuration of Ti = $[Ar]3{{d}^{2}}4{{s}^{2}}$
Atomic number of V = 23
Electronic configuration of V = $[Ar]3{{d}^{3}}4{{s}^{2}}$
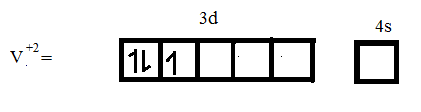
From the above discussion we can conclude that ${{V}^{+2}}$ has a higher number of unpaired electrons.
Note: To solve questions like this it is better to be through with the series and atomic number of the elements. When there is + ve charge on an element which means an electron is lost. If the element possesses -ve charge on it this means the element has gained an electron.
Complete answer:
The last electron in the atoms of these elements enters the d-sub shell belonging to the penultimate shell and are called transition metals.
The elements of first transition series are:
\[S{{c}_{21}},T{{i}_{22}},{{V}_{23}},C{{r}_{24}},M{{n}_{25}},F{{e}_{26}},C{{o}_{27}},N{{i}_{28}},C{{u}_{29}},Z{{n}_{30}}\]
i) Mn (+2,+3,+4,+5,+6,+7) possesses the highest number of oxidation states. This is because the electronic configuration of manganese is $[Ar]3{{d}^{5}}4{{s}^{2}}$and it possesses maximum number of electrons to share as well as lose.
(ii) Atomic number of Zn = 30
Electronic configuration is $[Ar]3{{d}^{10}}4{{s}^{2}}$.
It loses 2 electrons to form $Z{{n}^{+2}}$.

Atomic number of Cu = 29
Electronic configuration is $[Ar]3{{d}^{10}}$
It loses 2 electrons to form $C{{u}^{+2}}$.

$Z{{n}^{+2}}$ is diamagnetic nature and $C{{u}^{+2}}$ is paramagnetic nature. Since $Z{{n}^{+2}}$ is diamagnetic it is colourless. $C{{u}^{+2}}$ being paramagnetic in nature which has a unpaired electrons that absorbs radiation of certain wavelength and emits back light that imparts some colour.
(iii) Atomic number of Ti = 22
Electronic configuration of Ti = $[Ar]3{{d}^{2}}4{{s}^{2}}$

Atomic number of V = 23
Electronic configuration of V = $[Ar]3{{d}^{3}}4{{s}^{2}}$

From the above discussion we can conclude that ${{V}^{+2}}$ has a higher number of unpaired electrons.
Note: To solve questions like this it is better to be through with the series and atomic number of the elements. When there is + ve charge on an element which means an electron is lost. If the element possesses -ve charge on it this means the element has gained an electron.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




