
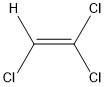
With which of the following does trichloroethene react to give a chiral product?
(a) \[B{r_2}\]
(b) \[HCl\]
(c) \[NaCN\left( {aq} \right)\]
(d) \[NaOH\left( {aq} \right)\]

Answer
562.8k+ views
Hint: A chiral compound is a compound which contains a \[s{p^3}\] hybridized carbon atom which is attached to four different carbon atoms. Such a compound shows optical isomerism.
Complete step by step answer:
A molecule is referred to as chiral if it is not superimposable on its mirror image. For example a pair of hands is also non superimposable on one another and is chiral. The molecule which exhibits such kind of property is said to possess geometrical isomerism.
A chiral molecule is said to show stereoisomerism which have the same molecular formula, same bonded atoms in the same sequence but differ in three dimensional arrangements in space. Molecules generally show two kinds of stereoisomers, one in enantiomers and the other is diastereomers. Enantiomers are non-superimposable mirror images of one another but diastereomers are not mirror images.
For example the following molecule is a chiral molecule which is attached to four different substituents.
The given substrate is trichloroethene which is an alkene compound. Both the carbon atoms are bonded by double bonds and are \[s{p^2}\] hybridized. It is an electron rich molecule and undergoes an electrophilic addition reaction to produce \[s{p^3}\] hybridized carbon atoms. Let us determine which of the given reagents can undergo electrophilic addition reaction with alkene to produce chiral product.
(a) \[B{r_2}\]. Molecular bromine is known as a good electrophile and undergoes additional reaction with electron rich substrates. Thus the product formed from the addition of bromine with the substrate trichloroethene is:
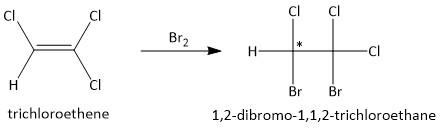
One of the two \[s{p^3}\] hybridized carbon atoms is attached to two four different substituents and is a chiral product.
(b) \[HCl\]. The addition of hydrochloric acid to alkene results in the formation of two compounds. The hydrogen and the chlorine attach itself to either of the two double bonded carbon atoms. Thus the two products are:
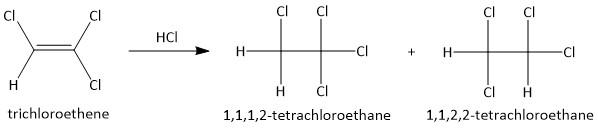
Here none of the products has an \[s{p^3}\] hybridized carbon atom which is attached to four different substituents. Hence none of the products are chiral.
(c) \[NaCN\left( {aq} \right)\]. It does take part in electrophilic addition reactions. It rather undergoes substitution reaction replacing any of the chlorine atoms from the molecule. Thus no \[s{p^3}\] hybridized compound is generated and the product is not chiral.
(d) \[NaOH\left( {aq} \right)\]. Here also no electrophilic addition reaction occurs. It rather undergoes substitution reaction replacing any of the chlorine atoms from the molecule. So no \[s{p^3}\] hybridized compound is generated and the product is not chiral.
Hence,option a is the correct answer.
Note: Chirality is the independent property of a molecule. The change of the plane of a polarized light in a polarimeter measures the amount of optical rotation of the chiral molecule.
Complete step by step answer:
A molecule is referred to as chiral if it is not superimposable on its mirror image. For example a pair of hands is also non superimposable on one another and is chiral. The molecule which exhibits such kind of property is said to possess geometrical isomerism.
A chiral molecule is said to show stereoisomerism which have the same molecular formula, same bonded atoms in the same sequence but differ in three dimensional arrangements in space. Molecules generally show two kinds of stereoisomers, one in enantiomers and the other is diastereomers. Enantiomers are non-superimposable mirror images of one another but diastereomers are not mirror images.
For example the following molecule is a chiral molecule which is attached to four different substituents.

The given substrate is trichloroethene which is an alkene compound. Both the carbon atoms are bonded by double bonds and are \[s{p^2}\] hybridized. It is an electron rich molecule and undergoes an electrophilic addition reaction to produce \[s{p^3}\] hybridized carbon atoms. Let us determine which of the given reagents can undergo electrophilic addition reaction with alkene to produce chiral product.
(a) \[B{r_2}\]. Molecular bromine is known as a good electrophile and undergoes additional reaction with electron rich substrates. Thus the product formed from the addition of bromine with the substrate trichloroethene is:

One of the two \[s{p^3}\] hybridized carbon atoms is attached to two four different substituents and is a chiral product.
(b) \[HCl\]. The addition of hydrochloric acid to alkene results in the formation of two compounds. The hydrogen and the chlorine attach itself to either of the two double bonded carbon atoms. Thus the two products are:

Here none of the products has an \[s{p^3}\] hybridized carbon atom which is attached to four different substituents. Hence none of the products are chiral.
(c) \[NaCN\left( {aq} \right)\]. It does take part in electrophilic addition reactions. It rather undergoes substitution reaction replacing any of the chlorine atoms from the molecule. Thus no \[s{p^3}\] hybridized compound is generated and the product is not chiral.
(d) \[NaOH\left( {aq} \right)\]. Here also no electrophilic addition reaction occurs. It rather undergoes substitution reaction replacing any of the chlorine atoms from the molecule. So no \[s{p^3}\] hybridized compound is generated and the product is not chiral.
Hence,option a is the correct answer.
Note: Chirality is the independent property of a molecule. The change of the plane of a polarized light in a polarimeter measures the amount of optical rotation of the chiral molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




