
How can I write a formula for potassium nitride?
Answer
548.4k+ views
Hint: We can see from the name of the compound that the compound potassium Nitride involves only 2 elements, potassium and Nitrogen. Thus, we can call it a binary compound involving a metal (potassium) from the group 1 of the periodic table, and a nonmetal (nitrogen) from the group 15 of the periodic table. However, Nitride is a negatively charged ion of nitrogen and potassium is a monovalent element, coming together to form a binary compound, hence the bond formed in ionic bond. The compound is neutral, and thus we shall accordingly set the number of cations and anions in the compound.
Complete Step by Step answer:
The given compound has the names of 2 elements involved. A metal and a nonmetal. But it does not involve any other element, so it can be called a binary compound. We know that the element potassium is from the group 1 of the periodic table, which consists of Alkali metals and is represented by the formula $ K $ . Also, the other element involved in the binary compound, Nitrogen is an element from the group 15 of the periodic table and is a non-metal element that is represented by the chemical formula $ N $ .
We also know that the noble gases/ rare gases are the most stable compounds in the periodic table and every other element tries to achieve the electronic configuration of the nearest noble gas possible to attain stability or tries to bond with other electrons in a compound to attain stability.
We can clearly see from the name of the compound that Nitride is an ion of nitrogen, that is formed by gaining 3 electrons to achieve the octet configuration of the nearest noble gas, Neon. Thus, by gaining 3 electrons from Nitrogen, Nitride is formed and represented by the formula, $ {N^{ - 3}} $ .similarly the group 1 element potassium with atomic number 19 is nearest to the noble gas Argon with atomic number 18, and hence potassium loses one electron to achieve a stable octet configuration of the nearest noble gas, Argon thus making the ion positively charged, $ {K^ + } $ .
Now we can see that the Nitride ion has negative charge $ - 3 $ and the element potassium has a positive charge $ + 1 $ . To form a compound the charges need to be neutralized and hence there should be 3 $ {K^ + } $ ions to bond with the nitride $ {N^{ - 3}} $ so that the compound is stable.
Thus, the formula of potassium nitride will be $ {K_3}N $ .
Note:
We can also use the Criss cross method to determine the formula of the compound.
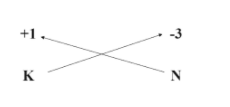
Thus, the formula of the compound potassium nitride will be $ {K_3}N $ .
Complete Step by Step answer:
The given compound has the names of 2 elements involved. A metal and a nonmetal. But it does not involve any other element, so it can be called a binary compound. We know that the element potassium is from the group 1 of the periodic table, which consists of Alkali metals and is represented by the formula $ K $ . Also, the other element involved in the binary compound, Nitrogen is an element from the group 15 of the periodic table and is a non-metal element that is represented by the chemical formula $ N $ .
We also know that the noble gases/ rare gases are the most stable compounds in the periodic table and every other element tries to achieve the electronic configuration of the nearest noble gas possible to attain stability or tries to bond with other electrons in a compound to attain stability.
We can clearly see from the name of the compound that Nitride is an ion of nitrogen, that is formed by gaining 3 electrons to achieve the octet configuration of the nearest noble gas, Neon. Thus, by gaining 3 electrons from Nitrogen, Nitride is formed and represented by the formula, $ {N^{ - 3}} $ .similarly the group 1 element potassium with atomic number 19 is nearest to the noble gas Argon with atomic number 18, and hence potassium loses one electron to achieve a stable octet configuration of the nearest noble gas, Argon thus making the ion positively charged, $ {K^ + } $ .
Now we can see that the Nitride ion has negative charge $ - 3 $ and the element potassium has a positive charge $ + 1 $ . To form a compound the charges need to be neutralized and hence there should be 3 $ {K^ + } $ ions to bond with the nitride $ {N^{ - 3}} $ so that the compound is stable.
Thus, the formula of potassium nitride will be $ {K_3}N $ .
Note:
We can also use the Criss cross method to determine the formula of the compound.

Thus, the formula of the compound potassium nitride will be $ {K_3}N $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




