
Write a note on Gabriel phthalimide synthesis.
What are biodegradable polymers and non-biodegradable polymers? Write one example of each.
Explain cationic detergents.
Answer
543.3k+ views
Hint: Gabriel phthalimide synthesis is the process of making primary amine from Phthalimide and ethanolic potassium hydroxide. Biodegradable polymers are decomposed naturally but non-biodegradable polymers are not decomposed naturally. Cationic detergents are used to remove impurities from clothes.
Complete answer:
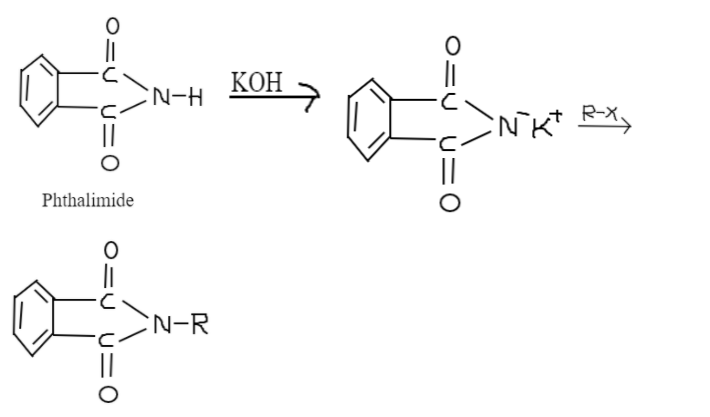
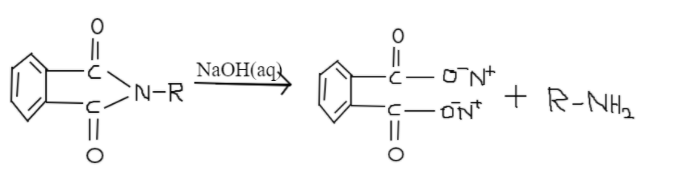
Gabriel phthalimide is used for the preparation of primary amines. When Phthalimide is treated with ethanolic potassium hydroxide, potassium salt of Phthalimide is produced. Heating it with alkyl halide and doing alkaline hydrolysis corresponding primary amine is produced. Aromatic primary amines cannot be prepared using this method. The reason behind that is aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Traditionally, potassium phthalimide was used in this reaction. This reaction is named after the German chemist Sigmund Gabriel as Gabriel phthalimide. This reaction is generalized to include the alkylation of sulfonamides and imides and also deportation to obtain amines. In this method, the sodium or potassium salt of phthalimide is N-alkylated with a primary alkyl halide to give the corresponding N-alkyl phthalimide.
The polymers which can decompose in a few days and are decomposed by the action of microorganisms are known as biodegradable polymers. Examples of biodegradable materials include vegetables and fruit peels, dead plants and animals, egg shells, chicken garden waste and paper materials etc.
The polymers which cannot decompose in a few days naturally are called non-biodegradable polymers. We have to follow a very long and hard process for decomposing non-biodegradable polymers. Examples of non-biodegradable polymers include plastics, polystyrene, metals, aluminum cans, toxic chemicals, paints, tires etc.
Cationic detergents contain a long-chain cation. They are responsible for their surface active properties. Examples of cationic detergents include quaternary ammonium compounds, benzalkonium chloride and cetyltrimethyl ammonium bromide.
Note:
Gabriel phthalimide is the reaction which is used to produce primary amines but not aromatic primary amines. We can decompose biodegradable polymers but not non-biodegradable polymers. Cationic detergents are used to remove surface impurities.
Complete answer:
Gabriel phthalimide is used for the preparation of primary amines. When Phthalimide is treated with ethanolic potassium hydroxide, potassium salt of Phthalimide is produced. Heating it with alkyl halide and doing alkaline hydrolysis corresponding primary amine is produced. Aromatic primary amines cannot be prepared using this method. The reason behind that is aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Traditionally, potassium phthalimide was used in this reaction. This reaction is named after the German chemist Sigmund Gabriel as Gabriel phthalimide. This reaction is generalized to include the alkylation of sulfonamides and imides and also deportation to obtain amines. In this method, the sodium or potassium salt of phthalimide is N-alkylated with a primary alkyl halide to give the corresponding N-alkyl phthalimide.


The polymers which can decompose in a few days and are decomposed by the action of microorganisms are known as biodegradable polymers. Examples of biodegradable materials include vegetables and fruit peels, dead plants and animals, egg shells, chicken garden waste and paper materials etc.
The polymers which cannot decompose in a few days naturally are called non-biodegradable polymers. We have to follow a very long and hard process for decomposing non-biodegradable polymers. Examples of non-biodegradable polymers include plastics, polystyrene, metals, aluminum cans, toxic chemicals, paints, tires etc.
Cationic detergents contain a long-chain cation. They are responsible for their surface active properties. Examples of cationic detergents include quaternary ammonium compounds, benzalkonium chloride and cetyltrimethyl ammonium bromide.
Note:
Gabriel phthalimide is the reaction which is used to produce primary amines but not aromatic primary amines. We can decompose biodegradable polymers but not non-biodegradable polymers. Cationic detergents are used to remove surface impurities.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




