
write a short note on Liebermann’s nitroso test.
Answer
510k+ views
Hint :aliphatic amines as well as aromatic mines are reactive organic compounds having nitrogen atoms derived from ammonia. Primary, secondary or tertiary amines show reactivity with various chemical reagents to produce different products. Amines are found among proteins, vitamins, alkaloids, hormones etc.
Complete Step By Step Answer:
Liebermann’s nitroso test is used to distinguish between primary, secondary and tertiary amines.
Reagent used in Liebermann’s nitroso test is freshly prepared nitrous acid $\left( {HN{O_2}} \right)$ which act as source of electrophilic nitrosonium ion $\left( {O = {N^ + }} \right)$ which react with amines.
Nitrous acid is highly unstable in nature due to this problem we have to prepare fresh reagent before performing the reaction.
Nitrous acid is prepared by treating sodium nitrite $\left( {NaN{O_2}} \right)$ with cold dilute hydrochloric acid or sulphuric acid.
$NaNO + HCl \to HONO + NaCl$
Secondary aliphatic or aromatic amines react slowly with nitrous acid in the cold condition to produce a yellow oily product known as nitroso amines.
${\left( {{C_2}{H_5}} \right)_2}NH + HONO \to {\left( {{C_2}{H_5}} \right)_2}N - N = O + {H_2}O$
Where ${\left( {{C_2}{H_5}} \right)_2}NH$- diethylamine
$HONO$- Nitrous acid
${\left( {{C_2}{H_5}} \right)_2}N - N = O$- N,N-Dimethyl nitrosamine (yellow oil)
Aromatic amine also reacts with nitrous acid to form yellow oil.
Yellow oil nitrosamine produced during the reaction gives green solution when it is warmed with phenol and concentrated sulphuric acid. On further dilution with water, the colour of the product changes from green to red.
When we add base sodium hydroxide to the red solution then its colour changes from greenish blue to violet.
This reaction is known as Liebermann’s nitroso test.
Note :
Primary amines react with nitrous acid rapidly to form aliphatic Diazonium salt. The salt produced is unstable in nature and undergoes decomposition to form alcohol and release nitrogen gas. This reaction is known as diazotization reaction.
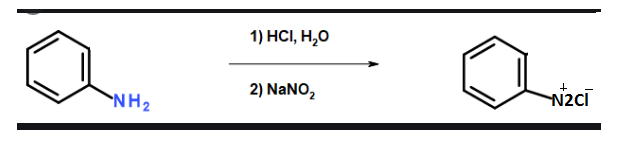
Complete Step By Step Answer:
Liebermann’s nitroso test is used to distinguish between primary, secondary and tertiary amines.
Reagent used in Liebermann’s nitroso test is freshly prepared nitrous acid $\left( {HN{O_2}} \right)$ which act as source of electrophilic nitrosonium ion $\left( {O = {N^ + }} \right)$ which react with amines.
Nitrous acid is highly unstable in nature due to this problem we have to prepare fresh reagent before performing the reaction.
Nitrous acid is prepared by treating sodium nitrite $\left( {NaN{O_2}} \right)$ with cold dilute hydrochloric acid or sulphuric acid.
$NaNO + HCl \to HONO + NaCl$
Secondary aliphatic or aromatic amines react slowly with nitrous acid in the cold condition to produce a yellow oily product known as nitroso amines.
${\left( {{C_2}{H_5}} \right)_2}NH + HONO \to {\left( {{C_2}{H_5}} \right)_2}N - N = O + {H_2}O$
Where ${\left( {{C_2}{H_5}} \right)_2}NH$- diethylamine
$HONO$- Nitrous acid
${\left( {{C_2}{H_5}} \right)_2}N - N = O$- N,N-Dimethyl nitrosamine (yellow oil)
Aromatic amine also reacts with nitrous acid to form yellow oil.
Yellow oil nitrosamine produced during the reaction gives green solution when it is warmed with phenol and concentrated sulphuric acid. On further dilution with water, the colour of the product changes from green to red.
When we add base sodium hydroxide to the red solution then its colour changes from greenish blue to violet.
This reaction is known as Liebermann’s nitroso test.
Note :
Primary amines react with nitrous acid rapidly to form aliphatic Diazonium salt. The salt produced is unstable in nature and undergoes decomposition to form alcohol and release nitrogen gas. This reaction is known as diazotization reaction.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




