
Write chemical reaction of glucose with HI
Answer
585.6k+ views
Hint: Glucose is a simple sugar with six carbon atoms and one aldehyde group. This monosaccharide has a chemical formula ${C_6}{H_{12}}{O_6}$. It is a carbohydrate and an important biomolecule that helps in the metabolism of the body.
Complete step by step answer:
Glucose is a widely available monosaccharide and is also known as dextrose and blood sugar. It is mainly manufactured by plants and most of the algae during the process of photosynthesis.
It is a white crystalline solid with melting point of ${146^ \circ }C$. When this glucose molecule is crystallized with cold water, it forms glucose monohydrate having molecular formula ${C_6}{H_{12}}{O_6}.{H_2}O$.
Now, let’s see the Haworth structure of glucose
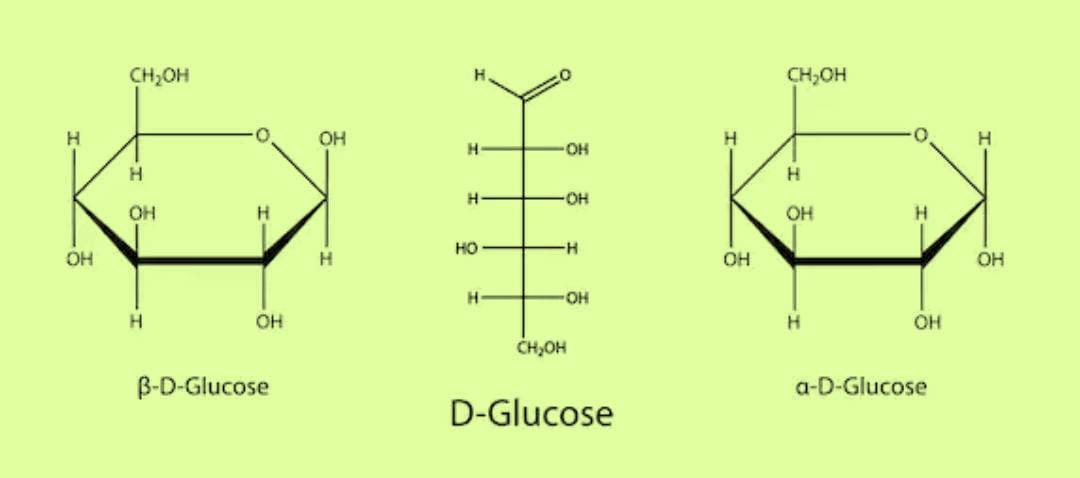
Haworth developed the hexagonal representations of glucose which resembled the heterocyclic pyran containing five carbon and one oxygen in the ring. He came up with the names alpha-D glucopyranose and $\beta - $D glucopyranose
Moreover, HI is a strong hydrogenating agent. On reacting with glucose, it replaces all the OH and carbonyl groups with hydrogen and forms n-hexane. The reaction is as shown:
${C_6}{H_{12}}{O_6} + HI \to {C_6}{H_{14}}$
The food that we consume comes from glucose or sugar. The prevalent sources of glucose are carbohydrates such as fruit, bread, cereals etc.
Note:
Glucose is used in the treatment of hypoglycemia i.e. low blood sugar. It is given to patients who are very sick and cannot eat, as it provides carbohydrates which is very essential for the body. It is also used in the treatment of increased potassium levels in the blood and as a precursor for the synthesis of a substance.
Complete step by step answer:
Glucose is a widely available monosaccharide and is also known as dextrose and blood sugar. It is mainly manufactured by plants and most of the algae during the process of photosynthesis.
It is a white crystalline solid with melting point of ${146^ \circ }C$. When this glucose molecule is crystallized with cold water, it forms glucose monohydrate having molecular formula ${C_6}{H_{12}}{O_6}.{H_2}O$.
Now, let’s see the Haworth structure of glucose

Haworth developed the hexagonal representations of glucose which resembled the heterocyclic pyran containing five carbon and one oxygen in the ring. He came up with the names alpha-D glucopyranose and $\beta - $D glucopyranose
Moreover, HI is a strong hydrogenating agent. On reacting with glucose, it replaces all the OH and carbonyl groups with hydrogen and forms n-hexane. The reaction is as shown:
${C_6}{H_{12}}{O_6} + HI \to {C_6}{H_{14}}$
The food that we consume comes from glucose or sugar. The prevalent sources of glucose are carbohydrates such as fruit, bread, cereals etc.
Note:
Glucose is used in the treatment of hypoglycemia i.e. low blood sugar. It is given to patients who are very sick and cannot eat, as it provides carbohydrates which is very essential for the body. It is also used in the treatment of increased potassium levels in the blood and as a precursor for the synthesis of a substance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




