
Write only reactions for the preparation of benzophenone from benzonitrile.
Answer
584.1k+ views
Hint: The preparation of benzophenone from benzonitrile is a three-step process.
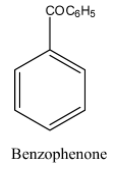
The structure of the benzophenone is as follows.
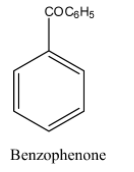
The structure of the benzonitrile is as follows.
Complete step by step solution:
Step-1:
In the step-1 hydrolysis of benzonitrile takes place. The chemical reaction of hydrolysis of benzonitrile is as follows.
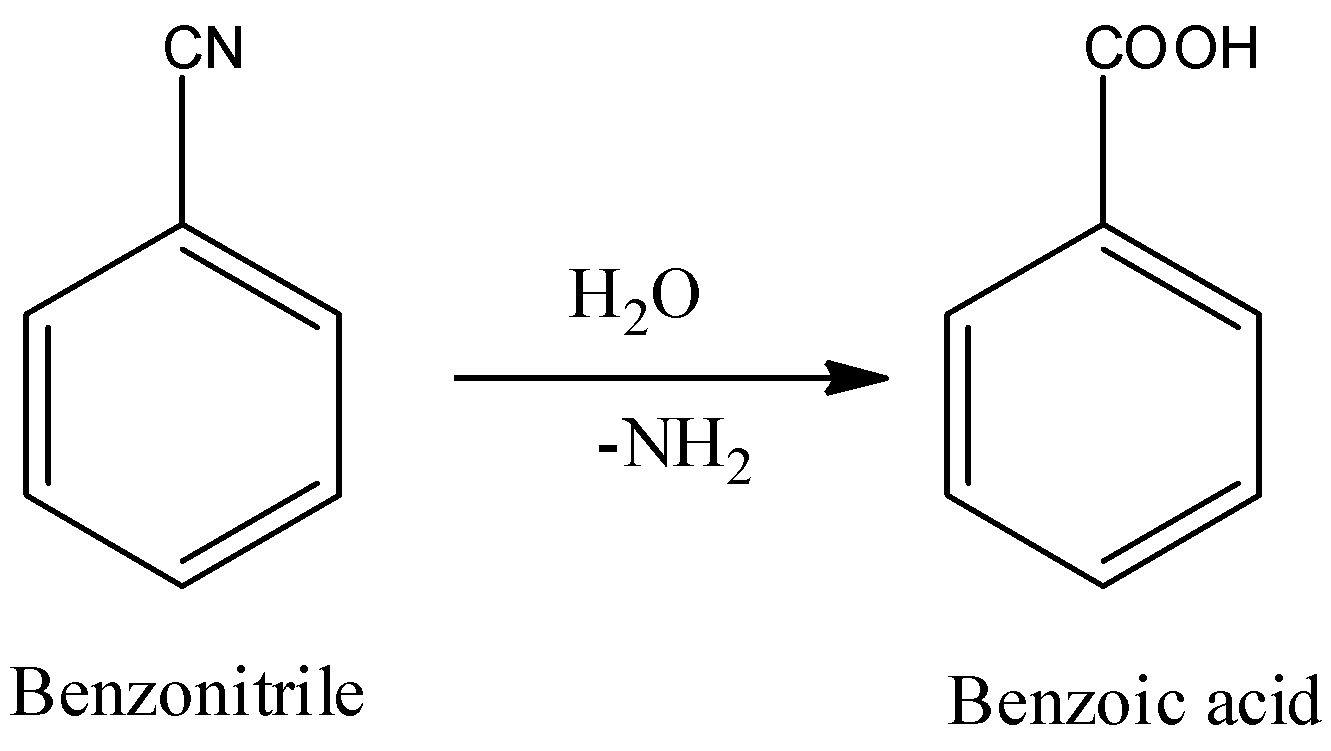
-In the above reaction benzonitrile undergoes hydrolysis and forms benzoic acid as the product.
-Generally, nitriles undergo hydrolysis and form carboxylic acids as the products.
Step-2:
In step-2 benzoic acid undergoes reaction with soda lime under heat and forms benzene as the product. The reaction of benzoic acid with soda lime is as follows.
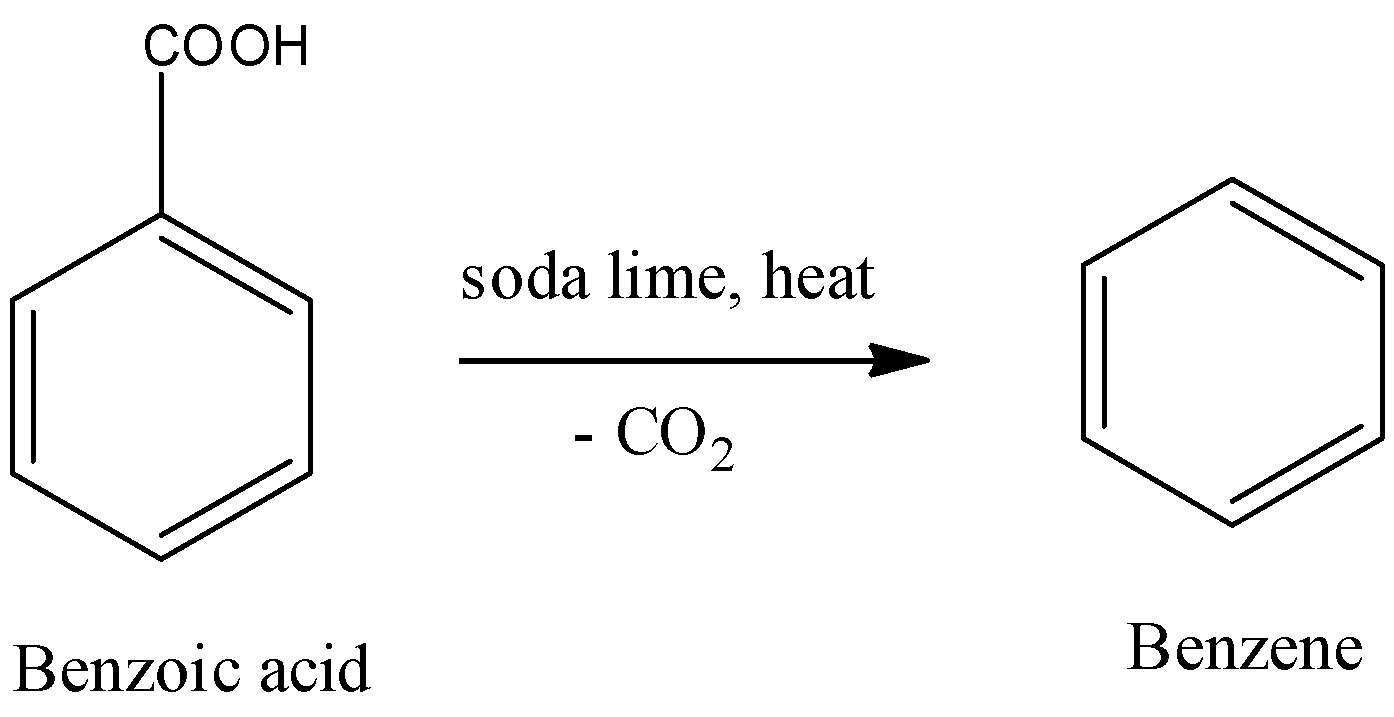
-The above reaction is called a decarboxylation reaction.
-A mixture of sodium hydroxide and calcium hydroxide is called soda lime.
Step-3:
-In step-3 benzene reacts with benzoyl chloride in presence of anhydrous aluminium chloride and forms benzophenone as the product.
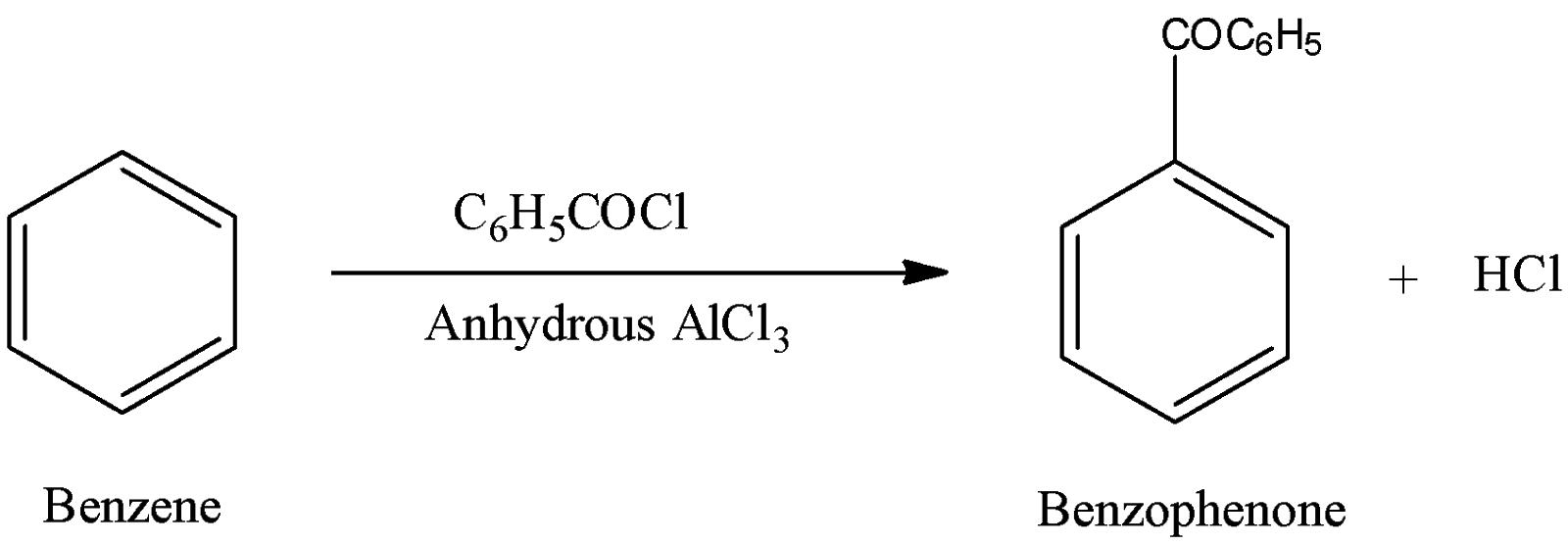
-The above reaction is an example of an electrophilic substitution reaction.
-The above reaction is called Friedel craft’s acylation reaction.
Additional information:
-Benzophenone is used as an additive for plastics.
-Perfumes, sunscreen lotions, nail polish, and shampoo contain benzophenone in their composition.
-The molecular formula of benzophenone is ${{C}_{13}}{{H}_{10}}O$.
-Benzophenone is a liquid and sweet-smelling organic compound in nature.
Note: Benzophenone in excess amounts is toxic. Generally, Cyanide compounds are called nitriles as per IUPAC nomenclature. We cannot prepare benzophenone from benzonitrile in a single-step reaction. Here we discussed the easiest way of preparation of benzophenone from benzonitrile.
The structure of the benzophenone is as follows.

The structure of the benzonitrile is as follows.

Complete step by step solution:
Step-1:
In the step-1 hydrolysis of benzonitrile takes place. The chemical reaction of hydrolysis of benzonitrile is as follows.

-In the above reaction benzonitrile undergoes hydrolysis and forms benzoic acid as the product.
-Generally, nitriles undergo hydrolysis and form carboxylic acids as the products.
Step-2:
In step-2 benzoic acid undergoes reaction with soda lime under heat and forms benzene as the product. The reaction of benzoic acid with soda lime is as follows.

-The above reaction is called a decarboxylation reaction.
-A mixture of sodium hydroxide and calcium hydroxide is called soda lime.
Step-3:
-In step-3 benzene reacts with benzoyl chloride in presence of anhydrous aluminium chloride and forms benzophenone as the product.

-The above reaction is an example of an electrophilic substitution reaction.
-The above reaction is called Friedel craft’s acylation reaction.
Additional information:
-Benzophenone is used as an additive for plastics.
-Perfumes, sunscreen lotions, nail polish, and shampoo contain benzophenone in their composition.
-The molecular formula of benzophenone is ${{C}_{13}}{{H}_{10}}O$.
-Benzophenone is a liquid and sweet-smelling organic compound in nature.
Note: Benzophenone in excess amounts is toxic. Generally, Cyanide compounds are called nitriles as per IUPAC nomenclature. We cannot prepare benzophenone from benzonitrile in a single-step reaction. Here we discussed the easiest way of preparation of benzophenone from benzonitrile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




