
Write preparation of
A.Nylon-6
B.Dextran
C.Buna-n
Answer
540k+ views
Hint:A molecule that can react with other molecules to form very large molecules having high molecular weights is known as a monomer. The high molecular weight molecules formed are known polymers. The monomer is a repeating unit of polymer.
Complete step-by-step answer:Preparation of nylon – 6:
Nylon – 6 is a type of nylon or polyamide. It has high strength and is very tough. It is formed by polycondensation of adipic acid and hexamethylenediamine under high pressure and high temperature conditions. Basically, it can be prepared by the ring opening polymerization of monomer caprolactam. The monomer used to prepare nylon-6 polymer is caprolactam.
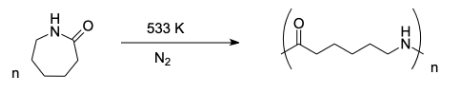
Preparation of Dextran:
Dextran is a polymer of poly acetic acid and polyglycolic acid. The polymer dextran has ester linkage. It is used as a friction modifier. It is prepared by an additional polymerization of glycolic acid and lactic acid. The monomers used to prepare dextran polymers are glycolic and lactic acid.
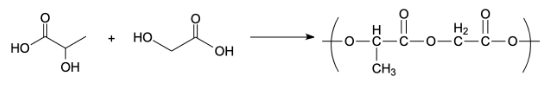
Preparation of Buna-n:
Buna-n is an example of a synthetic copolymer rubber. 1,3-Butadiene and acrylonitrile are copolymerized to form Buna-n. This copolymerization takes place in the presence of a peroxide catalyst.
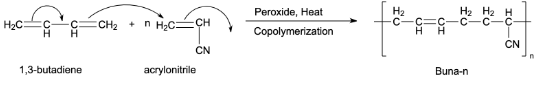
Note:Do not confuse Nylon-6 with nylon-6,6 which is prepared from adipic acid and hexamethylenediamine. Dextran is used in lubricants to reduce surface friction. Buna-n is also a copolymer which is prepared by the same method. It is prepared by the copolymerization between 1,3-butadiene and styrene.
Complete step-by-step answer:Preparation of nylon – 6:
Nylon – 6 is a type of nylon or polyamide. It has high strength and is very tough. It is formed by polycondensation of adipic acid and hexamethylenediamine under high pressure and high temperature conditions. Basically, it can be prepared by the ring opening polymerization of monomer caprolactam. The monomer used to prepare nylon-6 polymer is caprolactam.

Preparation of Dextran:
Dextran is a polymer of poly acetic acid and polyglycolic acid. The polymer dextran has ester linkage. It is used as a friction modifier. It is prepared by an additional polymerization of glycolic acid and lactic acid. The monomers used to prepare dextran polymers are glycolic and lactic acid.

Preparation of Buna-n:
Buna-n is an example of a synthetic copolymer rubber. 1,3-Butadiene and acrylonitrile are copolymerized to form Buna-n. This copolymerization takes place in the presence of a peroxide catalyst.

Note:Do not confuse Nylon-6 with nylon-6,6 which is prepared from adipic acid and hexamethylenediamine. Dextran is used in lubricants to reduce surface friction. Buna-n is also a copolymer which is prepared by the same method. It is prepared by the copolymerization between 1,3-butadiene and styrene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




