
Write reactions of the final alkylation product of aniline with excess of methyl iodide in the presence of sodium carbonate solution?
Answer
561k+ views
Hint: In order to find the alkylation product of aniline in presence of the excess of methyl iodide, we should be aware of the basic chemical reactions that are taking place. We should also have knowledge about why certain reagents are being used.
Complete step by step answer:
- Let us first understand about aniline. Aniline is an aromatic organic compound having a chemical formula of \[{C_6}{H_5}N{H_2}\]. Aniline is also known as aminobenzene or phenylamine. In its chemical formula it contains 6 carbon atoms, 7 hydrogen atoms and 1 nitrogen atom. Aniline is a yellow brownish oily liquid which has a fishy and musty odour. it is a highly flammable liquid.
- Now let us move to the given question. Aniline which is an aromatic primary amine will react with methyl iodide to give N, N-dimethylaniline. This N, N-dimethylaniline is a secondary aromatic amine.
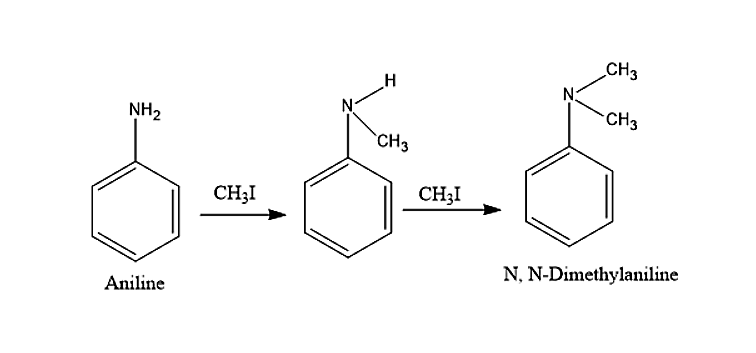
This N, N-Dimethylaniline will further react with excess methyl iodide and sodium carbonate to give a final product N, N, N- Trimethylanilinium carbonate.
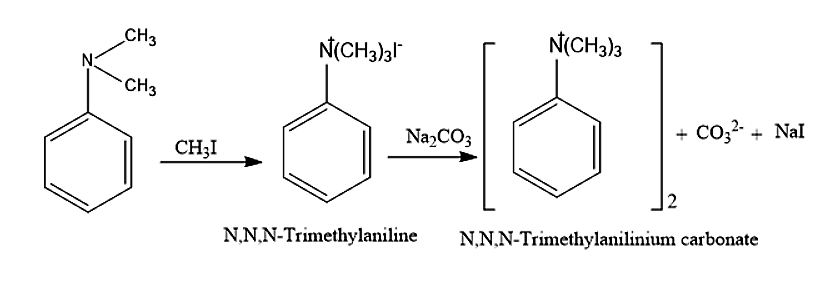
Therefore, the final alkylation product of aniline is N, N, N- Trimethylanilinium carbonate.
Note: Aniline is having various uses such as:
- It is used in the rubber industry for making car tyres, gloves and balloons.
- It is used in production of drugs such as paracetamol, acetaminophen and Tylenol.
- It is used in the agricultural industry for producing fungicides and pesticides.
- it is used as a dyeing agent in the manufacture of jeans.
Complete step by step answer:
- Let us first understand about aniline. Aniline is an aromatic organic compound having a chemical formula of \[{C_6}{H_5}N{H_2}\]. Aniline is also known as aminobenzene or phenylamine. In its chemical formula it contains 6 carbon atoms, 7 hydrogen atoms and 1 nitrogen atom. Aniline is a yellow brownish oily liquid which has a fishy and musty odour. it is a highly flammable liquid.
- Now let us move to the given question. Aniline which is an aromatic primary amine will react with methyl iodide to give N, N-dimethylaniline. This N, N-dimethylaniline is a secondary aromatic amine.

This N, N-Dimethylaniline will further react with excess methyl iodide and sodium carbonate to give a final product N, N, N- Trimethylanilinium carbonate.

Therefore, the final alkylation product of aniline is N, N, N- Trimethylanilinium carbonate.
Note: Aniline is having various uses such as:
- It is used in the rubber industry for making car tyres, gloves and balloons.
- It is used in production of drugs such as paracetamol, acetaminophen and Tylenol.
- It is used in the agricultural industry for producing fungicides and pesticides.
- it is used as a dyeing agent in the manufacture of jeans.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




