
Write resonance structure of $C{H_2} = CH - CHO$. Indicate relative stability of the contributing structures.
Answer
510.3k+ views
Hint: We need to know that the stability of any atom will be the maximum if it has a complete octet. Octet is the most stable configuration. Also, greater the number of covalent bonds, it’ll have more stability, as more atoms will have an octet.
Complete answer:
The factors that affect the stability of a species are:
Number of Covalent Bonds: More the number of covalent bonds, more will be the atoms with octet, more will be the stability.
Formal Charge: The species should have the least formal charge to be stable.
Charge Separation: The species should have least charge separation to be stable.
Charge on Atoms: The species with negative charge on electropositive atom and positive charge on electronegative atom will be least stable.
The given compound is Prop-2-ene-1-al. The resonance in this compound can be shown as:
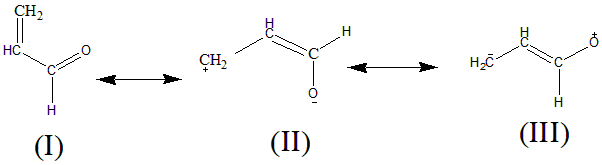
In here, Structure (I) will have the highest stability as it is a neutral molecule and has the maximum number of covalent bonds. All the atoms have a complete octet; hence it’ll be most stable among all.
Oxygen is more electronegative than Carbon. In structure (II) Oxygen has a negative charge (electronegative atom) and carbon has a positive charge (electropositive atom) which makes it less stable than (I) due to the charge separation.
In structure (III) the electropositive atom Carbon has negative charge. Carbon becomes unstable because of the negative charge, and oxygen which is more electronegative has a positive charge. Hence this species is the most Unstable of all.
The order of stability can be given as: I > II > III
Note:
Always remember that Neutral Molecules will have the maximum stability than any charged species. We need to remember that the neutral species are those which don't have any positive or negative charge in their rest state.
Complete answer:
The factors that affect the stability of a species are:
Number of Covalent Bonds: More the number of covalent bonds, more will be the atoms with octet, more will be the stability.
Formal Charge: The species should have the least formal charge to be stable.
Charge Separation: The species should have least charge separation to be stable.
Charge on Atoms: The species with negative charge on electropositive atom and positive charge on electronegative atom will be least stable.
The given compound is Prop-2-ene-1-al. The resonance in this compound can be shown as:

In here, Structure (I) will have the highest stability as it is a neutral molecule and has the maximum number of covalent bonds. All the atoms have a complete octet; hence it’ll be most stable among all.
Oxygen is more electronegative than Carbon. In structure (II) Oxygen has a negative charge (electronegative atom) and carbon has a positive charge (electropositive atom) which makes it less stable than (I) due to the charge separation.
In structure (III) the electropositive atom Carbon has negative charge. Carbon becomes unstable because of the negative charge, and oxygen which is more electronegative has a positive charge. Hence this species is the most Unstable of all.
The order of stability can be given as: I > II > III
Note:
Always remember that Neutral Molecules will have the maximum stability than any charged species. We need to remember that the neutral species are those which don't have any positive or negative charge in their rest state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




