
Write resonance structures for $CO_3^{2 - }$ and $HCO_3^ - $ ions.
Answer
583.8k+ views
Hint: Resonance is a way of describing bonding in certain molecules or ions by the combination of several contributing structures (or forms also variously known as resonance structures or canonical structures) into a resonance hybrid (or hybrid structures) in valence bond theory.
Complete step by step answer:
It has particular value for describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
The resonance structure of carbonate ion and bicarbonate ion along with resonance hybrid are shown below.
$CO_3^{2 - }$
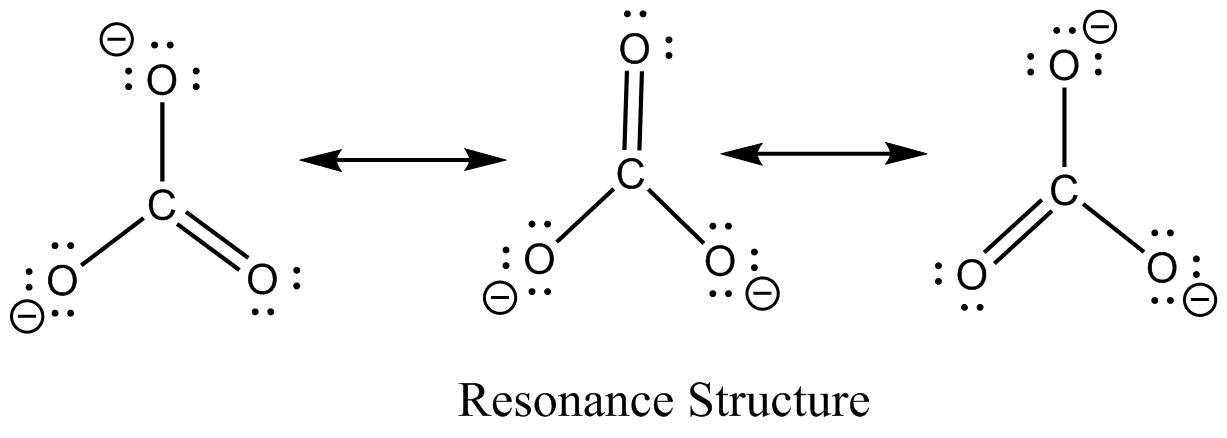
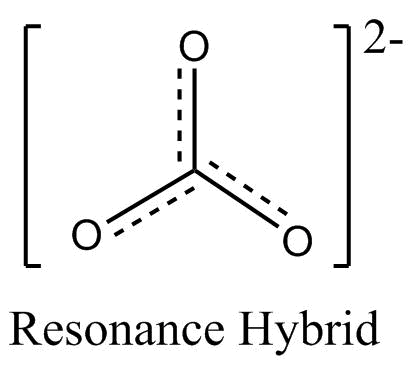
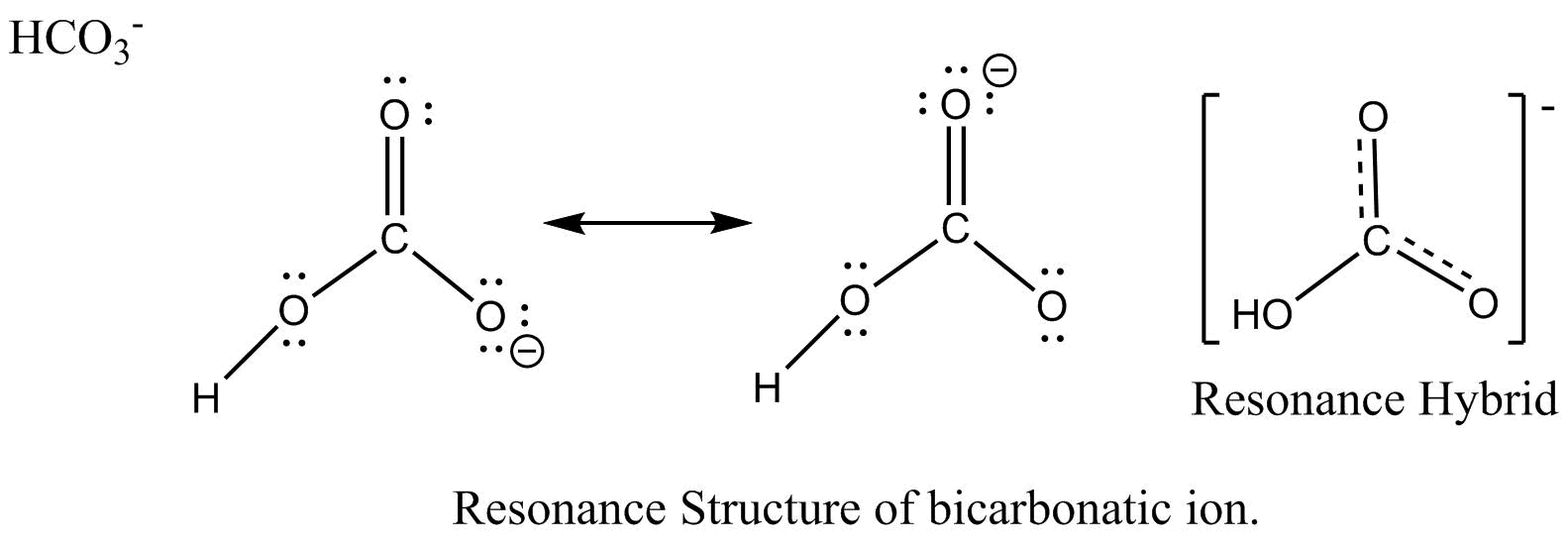
Additional Information:
A resonance form is a different way of drawing a Lewis dot structure for a given compound. The Lewis structures which are equivalent are called resonance forms. They are used when there is more than one way to place double bonds and ion pairs of atoms.
They arise when there are more than one way to draw a Lewis dot diagram that satisfies the octet rule.
Resonance structures do not exist in real life l, they just show possible structures for a compound They are not in equilibrium with each other. They are not isomers as Isomers have different arrangements of both atoms and electrons whereas Resonance forms differ only in arrangement of electrons.
Resonance structures are much better than Lewis dot structures because in them bonding in molecules is clearly shown .
Note:
Formal charges are used in chemistry to determine the location of a charge in a molecule and determine how good of a Lewis structure it will be remembered, the best Resonance structure is the one with the least formal charges are very important.
Complete step by step answer:
It has particular value for describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
The resonance structure of carbonate ion and bicarbonate ion along with resonance hybrid are shown below.
$CO_3^{2 - }$



Additional Information:
A resonance form is a different way of drawing a Lewis dot structure for a given compound. The Lewis structures which are equivalent are called resonance forms. They are used when there is more than one way to place double bonds and ion pairs of atoms.
They arise when there are more than one way to draw a Lewis dot diagram that satisfies the octet rule.
Resonance structures do not exist in real life l, they just show possible structures for a compound They are not in equilibrium with each other. They are not isomers as Isomers have different arrangements of both atoms and electrons whereas Resonance forms differ only in arrangement of electrons.
Resonance structures are much better than Lewis dot structures because in them bonding in molecules is clearly shown .
Note:
Formal charges are used in chemistry to determine the location of a charge in a molecule and determine how good of a Lewis structure it will be remembered, the best Resonance structure is the one with the least formal charges are very important.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




