
Write the chemical formulae of the following by criss-cross method:
A.Magnesium chloride
B.Calcium oxide
C.Copper nitrate
D.Aluminium chloride
E.Potassium nitrate
Answer
530.8k+ views
Hint: We know that chemical formulas can also be written using criss-cross method. In the criss-cross method, the numerical value of the ion charge of the two atoms are crossed over, which becomes the subscript of the other ion. Using this technique, we will write the chemical formula of the given compounds.
Complete step by step answer:
Let’s us discuss about the given compound as,
A.Magnesium chloride
We have to remember that the atomic number of Magnesium is $12$ and has a valency of 2. It means it has two electrons in the outermost shell for bonding. The atomic number of chlorine is $17$ and has 7 electrons in the outermost shell. It means it just needs one more atom for bonding. Hence, we will use atoms of chlorine to bond with one atom of magnesium.
We can apply the criss-cross method for this compound as,
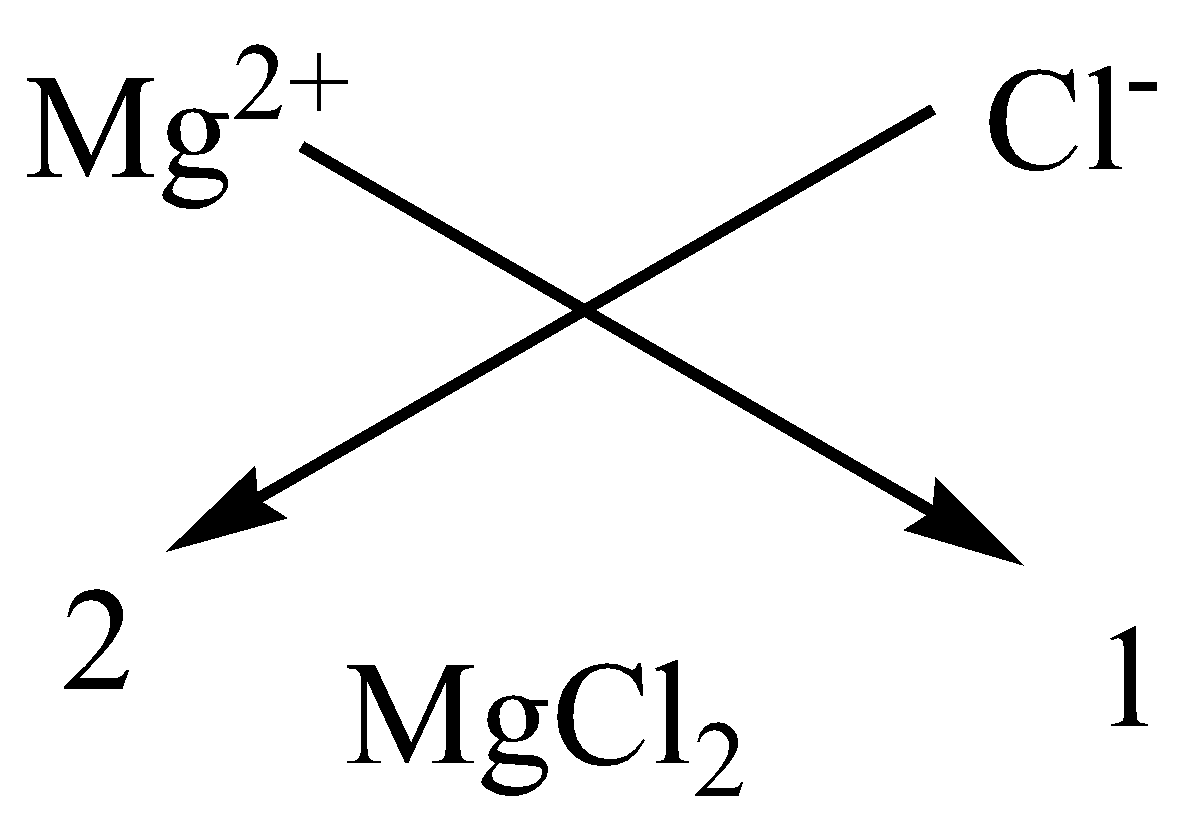
Therefore, the chemical formula of magnesium chloride is $MgC{l_2}$.
B.Calcium oxide
We have to know that the atomic number of calcium $20$ and has a valency of 2, it means it has 2 two atoms in the outermost shell for bonding. The atomic number of Oxygen is $8$ and has a valency of 2, it has 6 atoms in the outermost shell, it needs more 2 to complete the octet. Hence, we need one calcium atom to bond with one oxygen atom.
We can apply the criss-cross method for this compound as,
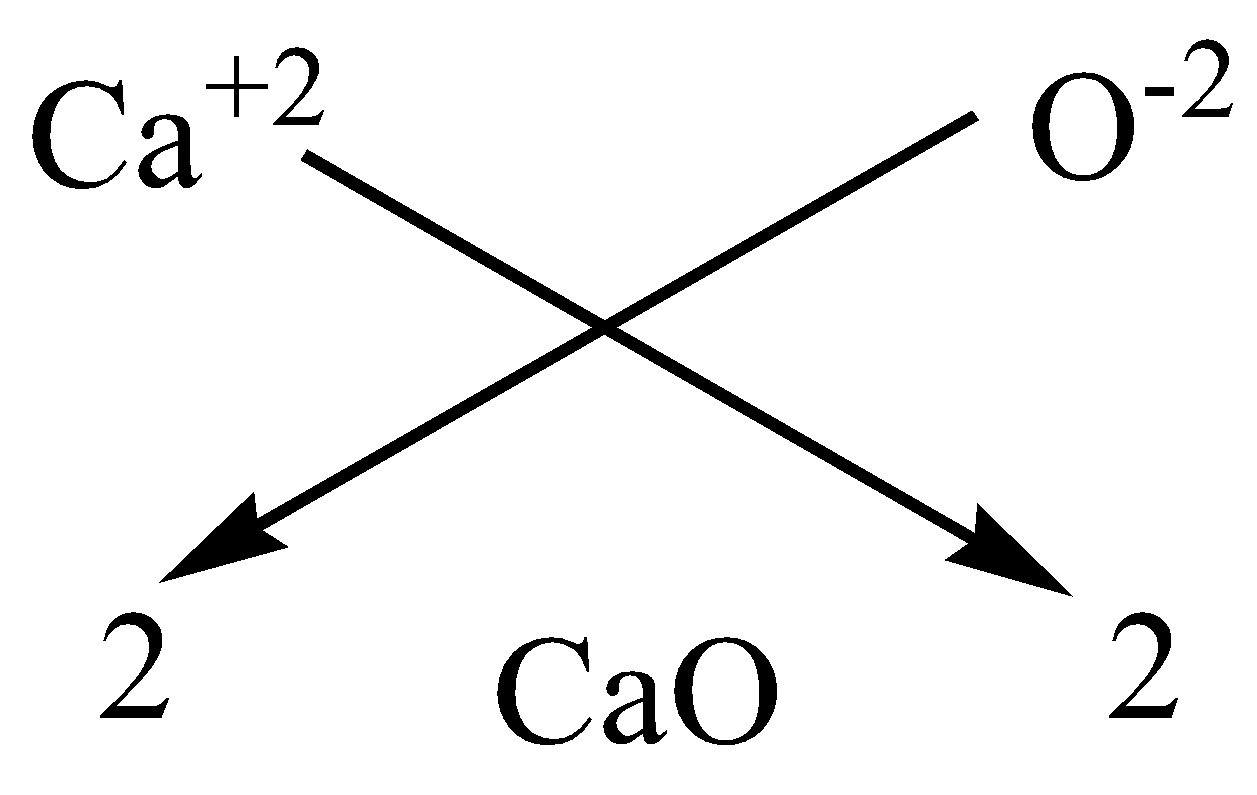
Therefore, the chemical formula of magnesium chloride is $CaO$.
C.Copper nitrate
We have to know that the atomic number of copper is $29$ and has two atoms in the outermost shell for bonding. While a nitrate molecule has only one valence electron. We need 2 nitrate molecules to satisfy the valency of 1 copper atom.
We can apply the criss-cross method for this compound as,
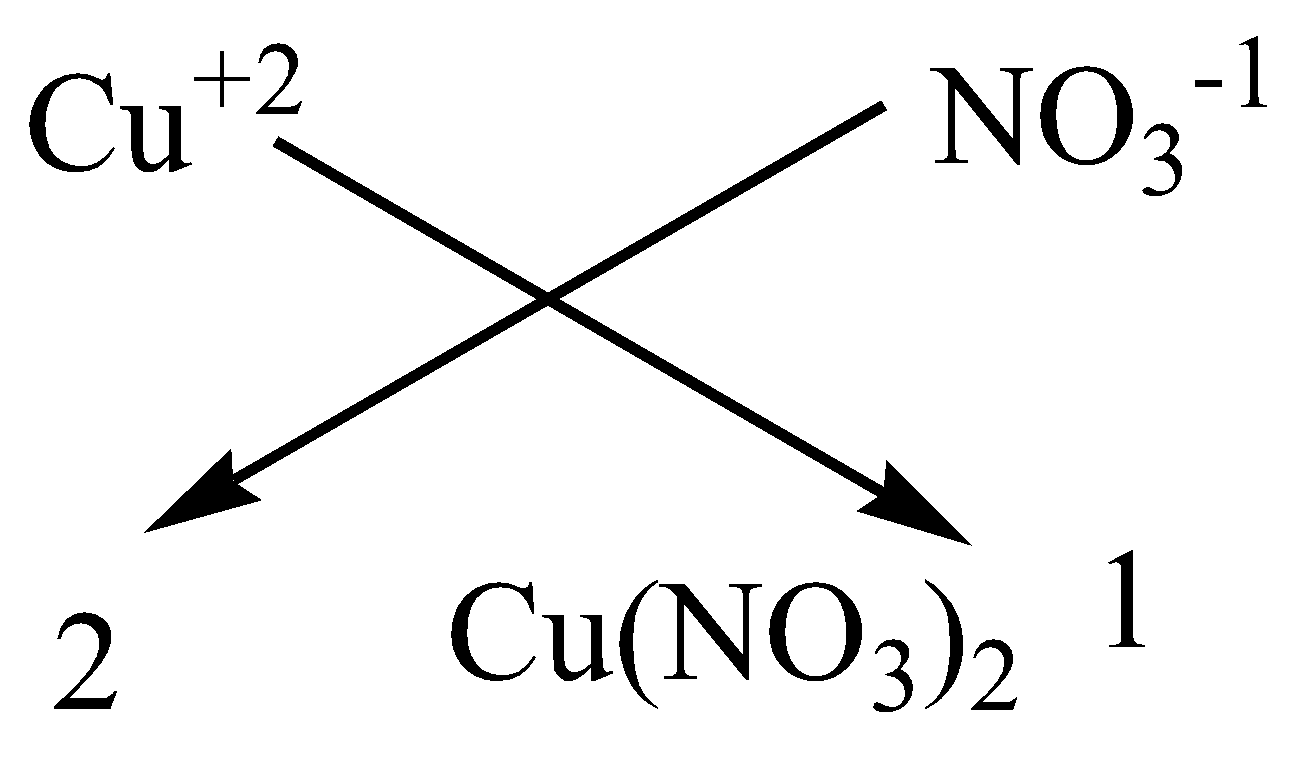
Therefore, the chemical formula of magnesium chloride is $Cu{\left( {N{O_3}} \right)_2}$.
D.Aluminium chloride
We have to know that the atomic number of aluminium is 13 and has a valency of 3 atoms and chlorine atom has a valency of 1. Since it has 7 electrons in the outermost shell. Thus, we need 3 chlorine atoms to satisfy the valency of 1 aluminium atom.
We can apply the criss-cross method for this compound as,
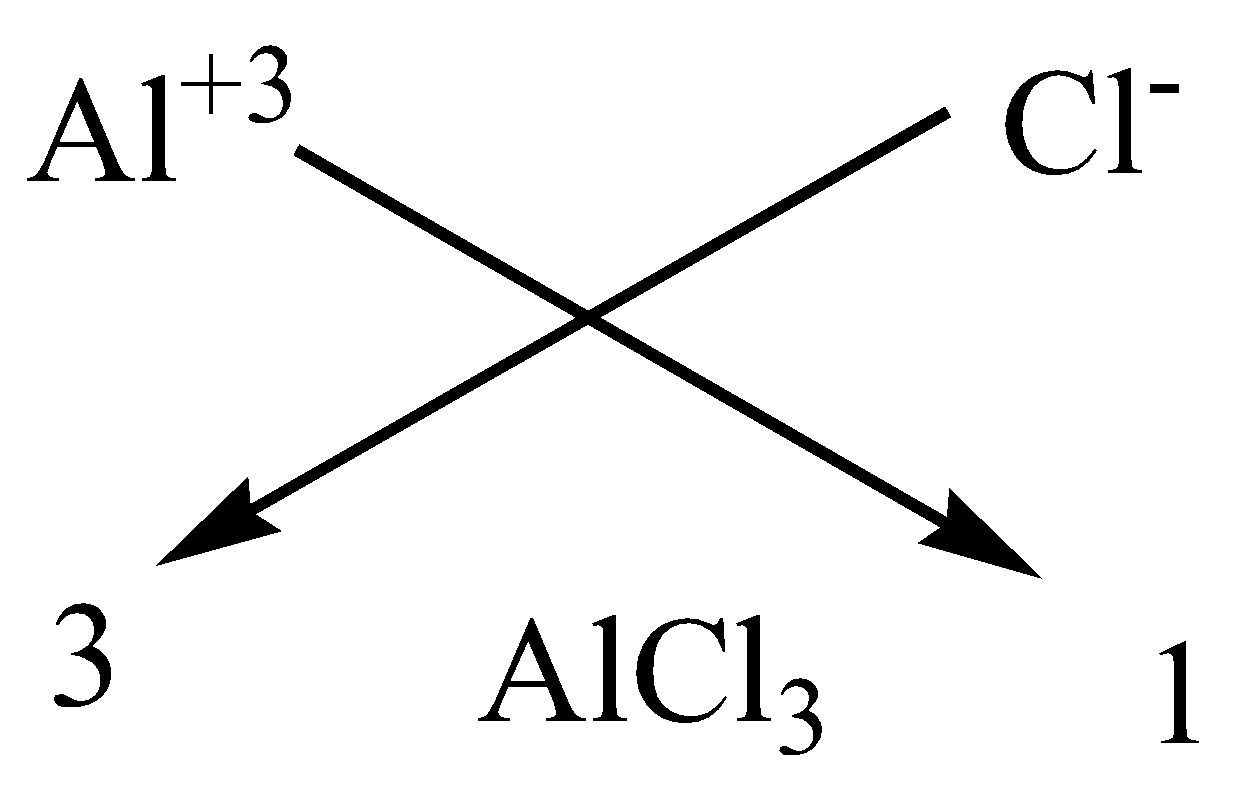
Therefore, the chemical formula of magnesium chloride is $AlC{l_3}$.
E.Potassium nitrate
We have to remember that the atomic number of potassium is 19 and has a valency of 1 and nitrate also has a valency of 1, since it needs one more atom to complete its octet. Hence, we need only one molecule of nitrate for one atom of potassium.
We can apply the criss-cross method for this compound as,
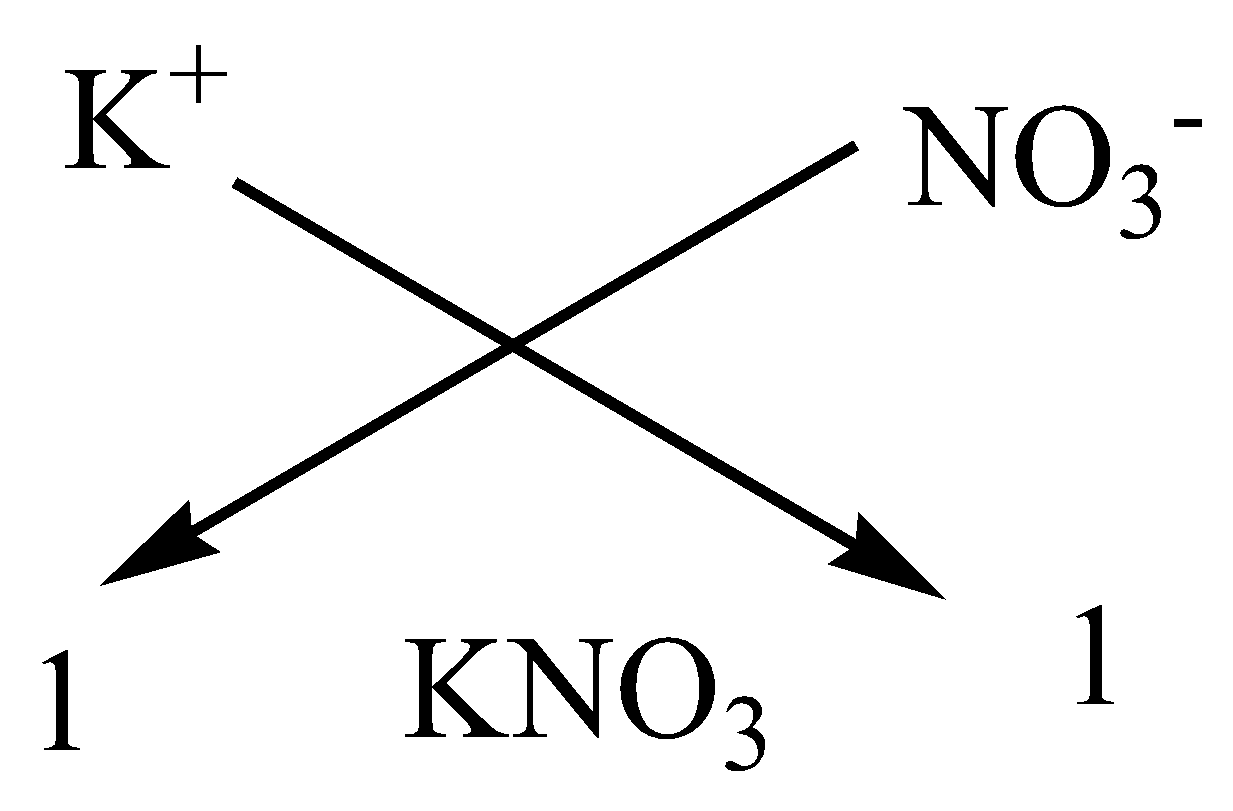
Therefore, the chemical formula of magnesium chloride is $KN{O_3}$.
Note: As we know that the criss-cross method is the most efficient way to write the correct chemical formula of the molecule. It is generally used for finding out the formula of a bonding of a metal with a non-metal to form ionic bonds. Signs of the two ions are dropped, the ion value is crossed which becomes the subscript of the crossed atoms.
Complete step by step answer:
Let’s us discuss about the given compound as,
A.Magnesium chloride
We have to remember that the atomic number of Magnesium is $12$ and has a valency of 2. It means it has two electrons in the outermost shell for bonding. The atomic number of chlorine is $17$ and has 7 electrons in the outermost shell. It means it just needs one more atom for bonding. Hence, we will use atoms of chlorine to bond with one atom of magnesium.
We can apply the criss-cross method for this compound as,

Therefore, the chemical formula of magnesium chloride is $MgC{l_2}$.
B.Calcium oxide
We have to know that the atomic number of calcium $20$ and has a valency of 2, it means it has 2 two atoms in the outermost shell for bonding. The atomic number of Oxygen is $8$ and has a valency of 2, it has 6 atoms in the outermost shell, it needs more 2 to complete the octet. Hence, we need one calcium atom to bond with one oxygen atom.
We can apply the criss-cross method for this compound as,

Therefore, the chemical formula of magnesium chloride is $CaO$.
C.Copper nitrate
We have to know that the atomic number of copper is $29$ and has two atoms in the outermost shell for bonding. While a nitrate molecule has only one valence electron. We need 2 nitrate molecules to satisfy the valency of 1 copper atom.
We can apply the criss-cross method for this compound as,

Therefore, the chemical formula of magnesium chloride is $Cu{\left( {N{O_3}} \right)_2}$.
D.Aluminium chloride
We have to know that the atomic number of aluminium is 13 and has a valency of 3 atoms and chlorine atom has a valency of 1. Since it has 7 electrons in the outermost shell. Thus, we need 3 chlorine atoms to satisfy the valency of 1 aluminium atom.
We can apply the criss-cross method for this compound as,

Therefore, the chemical formula of magnesium chloride is $AlC{l_3}$.
E.Potassium nitrate
We have to remember that the atomic number of potassium is 19 and has a valency of 1 and nitrate also has a valency of 1, since it needs one more atom to complete its octet. Hence, we need only one molecule of nitrate for one atom of potassium.
We can apply the criss-cross method for this compound as,

Therefore, the chemical formula of magnesium chloride is $KN{O_3}$.
Note: As we know that the criss-cross method is the most efficient way to write the correct chemical formula of the molecule. It is generally used for finding out the formula of a bonding of a metal with a non-metal to form ionic bonds. Signs of the two ions are dropped, the ion value is crossed which becomes the subscript of the crossed atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




