
Write the chemical reaction showing complete combustion of the organic substance formed in sodium hydroxide and chloroethane?
Answer
493.5k+ views
Hint: This question is a tricky one. In simple words, you must first write the reaction between sodium hydroxide and chloroethane and you will get some product. Now, this product will undergo complete combustion. The reaction between $ NaOH $ and $ {C_2}{H_5}Cl $ is a $ {S_N}2 $ reaction, where the nucleophile will attack the alkyl halide to give the product. On complete combustion, gas and water are produced as products.
Complete Step By Step Answer:
According to the question first, we have to find the product of the reaction between sodium hydroxide and chloroethane and then we have to show the complete combustion reaction of that product formed by reacting the two.
The reaction between sodium hydroxide $ \left( {NaOH} \right) $ and chloroethane $ \left( {{C_2}{H_5}Cl} \right) $ is a $ {S_N}2 $ reaction that is bimolecular nucleophilic substitution reaction. In this reaction the breaking of one bond and the formation of another bond occurs simultaneously. During the reaction a transition state is achieved. The reaction between $ NaOH $ and $ {C_2}{H_5}Cl $ takes place in the following manner:
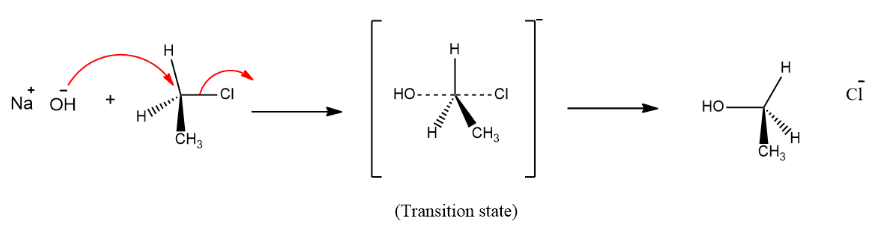
As we can see in the reaction that the product formed is ethanol $ \left( {{C_2}{H_5}OH} \right) $ .
Now we have to show how this organic product, $ {C_2}{H_5}OH $ undergoes complete combustion. A complete combustion reaction is the reaction of fuel with oxygen. In this reaction, a large amount of heat is given out. When ethanol undergoes complete combustion, it reacts with oxygen and forms the products carbon dioxide and water. Thus the reaction for the complete combustion of ethanol is:
$ {C_2}{H_5}OH + 3{O_2} \to 2C{O_2} + 3{H_2}O $ .
Note:
Combustion is a chemical process where a substance reacts rapidly with oxygen to form the product. The burning of coal is one of the examples of complete combustion. There is also something called incomplete combustion. In this reaction, oxygen availability is less for the fuel to completely react to produce carbon dioxide and water. The complete combustion reaction always occurs between fuel and oxygen. The reaction is the same as shown above and the products formed are carbon dioxide and water in most cases.
Complete Step By Step Answer:
According to the question first, we have to find the product of the reaction between sodium hydroxide and chloroethane and then we have to show the complete combustion reaction of that product formed by reacting the two.
The reaction between sodium hydroxide $ \left( {NaOH} \right) $ and chloroethane $ \left( {{C_2}{H_5}Cl} \right) $ is a $ {S_N}2 $ reaction that is bimolecular nucleophilic substitution reaction. In this reaction the breaking of one bond and the formation of another bond occurs simultaneously. During the reaction a transition state is achieved. The reaction between $ NaOH $ and $ {C_2}{H_5}Cl $ takes place in the following manner:

As we can see in the reaction that the product formed is ethanol $ \left( {{C_2}{H_5}OH} \right) $ .
Now we have to show how this organic product, $ {C_2}{H_5}OH $ undergoes complete combustion. A complete combustion reaction is the reaction of fuel with oxygen. In this reaction, a large amount of heat is given out. When ethanol undergoes complete combustion, it reacts with oxygen and forms the products carbon dioxide and water. Thus the reaction for the complete combustion of ethanol is:
$ {C_2}{H_5}OH + 3{O_2} \to 2C{O_2} + 3{H_2}O $ .
Note:
Combustion is a chemical process where a substance reacts rapidly with oxygen to form the product. The burning of coal is one of the examples of complete combustion. There is also something called incomplete combustion. In this reaction, oxygen availability is less for the fuel to completely react to produce carbon dioxide and water. The complete combustion reaction always occurs between fuel and oxygen. The reaction is the same as shown above and the products formed are carbon dioxide and water in most cases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




