
Write the electron dot structure of the ethyne molecule $\text{ }{{\text{C}}_{\text{2}}}{{\text{H}}_{2}}\text{ }$ .
Answer
573.3k+ views
Hint: Lewis dot structure or the electron dot structure is a way of representation of valence electrons in the atom. These valence electrons are represented by the dot around the symbol of the element. The total number of valence electrons is equal to the number of dots in the structure. To determine the electron dot structure, determine the central atom (atoms which have high valence number and high electronegativity) and surrounded by the atom which has a low electronegativity. The ethyne has a molecular formula as $\text{ }{{\text{C}}_{\text{2}}}{{\text{H}}_{2}}\text{ }$ or $\,\text{CH}\equiv \text{CH }$ .
Complete answer:
Let's draw a Lewis dot structure for ethane molecules. Ethyne is a saturated hydrocarbon. From its name it is clear that ethyne has two carbon atoms. Each carbon is $\,\text{sp }$ hybridized and the molecular structure is$\text{ }{{\text{C}}_{\text{2}}}{{\text{H}}_{2}}\text{ }$.
Follows the below step to determine the Lewis dot structure of the ethyne
Step 1) the first step determines the total number of valence electrons in the carbon atom. the electronic configuration of carbon is as follows,
$\text{ C = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{2}}}\text{ }$
Here, carbon has a 4 valence electron.
Similarly, the electronic configuration of hydrogen is, $\text{ H = 1}{{\text{s}}^{\text{1}}}\text{ }$
Here, carbon has a 1valence electron and requires one electron to attain the nearest noble gas configuration.
Step 2) In this step, draw a skeleton structure of the ethyne molecule. Connect all atoms through the single bond. The central atom will be the atom which has the highest number of valence electrons. Therefore, ethyne carbon is a central carbon atom. The skeleton would involve two carbon atoms surrounded by the two carbon atoms. The skeleton structure of ethyne is,
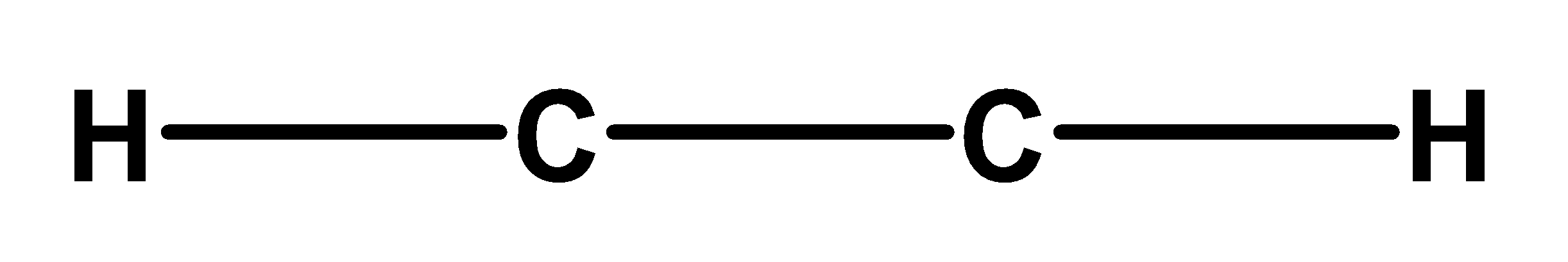
Step 3) In this step we will add all pairs of electrons on the atom. Each carbon atom has 4 valence electrons. Thus total valence electrons from two carbon atoms are $\begin{align}
& \text{ Total valence }{{\text{e}}^{-}}\text{ = 2}\times \text{(V}\text{.E}\text{. of C ) + 2}\times \text{(V}\text{.E}\text{. of H ) } \\
& \Rightarrow \text{ 2}\times \text{ 4 + 2}\times \text{1 = 10 } \\
\end{align}$
Thus we need to place 10 valence electrons on the ethyne molecule. Here six electrons are involved in the bond pairs. We need to place 4 electrons on the skeleton. The hydrogen atom has completed its octet. But each carbon requires 2 more electrons to complete its octet. thus add 4 electrons on the two carbon atoms to complete its octet. It is as shown below,

Ethyne structure can also be represented as,
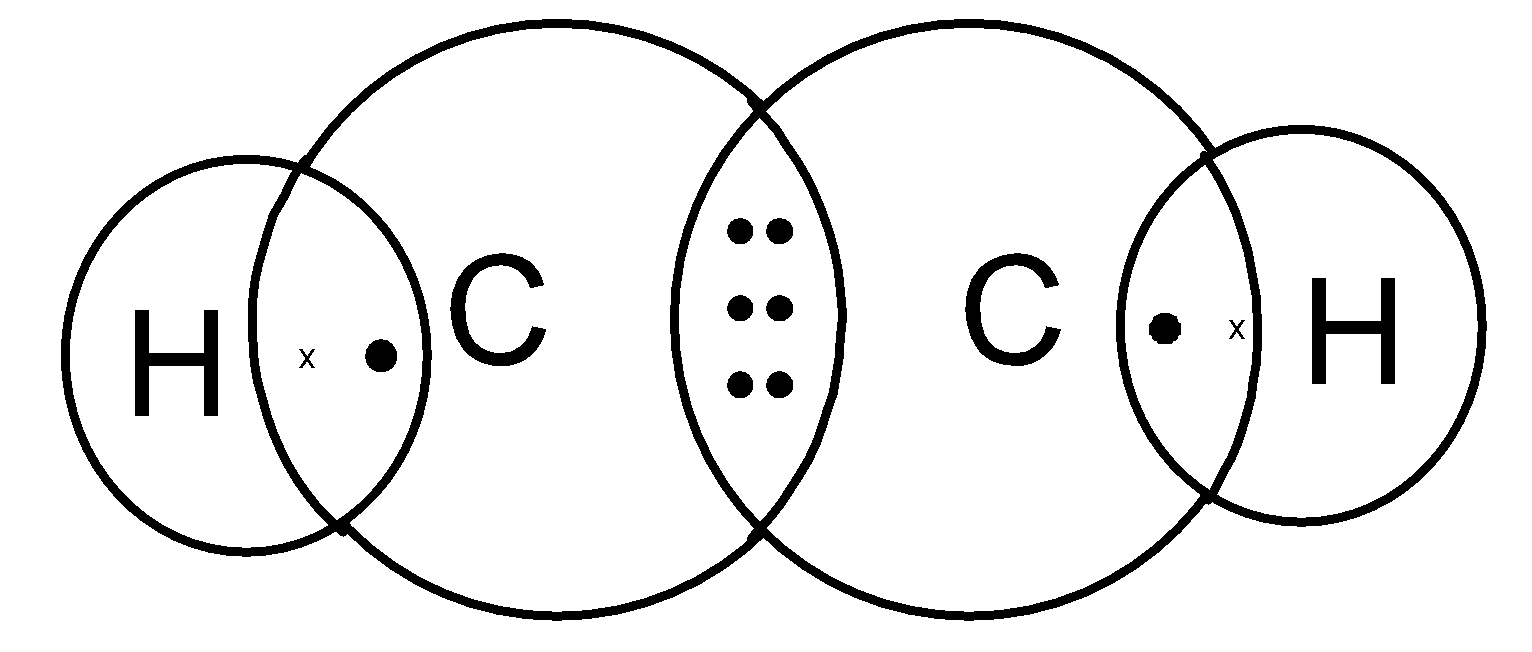
Note: The Lewis dot structure does not explain the geometry of molecules or how the Bonds are formed, or how the electrons are shared between the atoms. The Lewis dot structure is a simple and limited theory based on the electronic structure and valence numbers.
Complete answer:
Let's draw a Lewis dot structure for ethane molecules. Ethyne is a saturated hydrocarbon. From its name it is clear that ethyne has two carbon atoms. Each carbon is $\,\text{sp }$ hybridized and the molecular structure is$\text{ }{{\text{C}}_{\text{2}}}{{\text{H}}_{2}}\text{ }$.
Follows the below step to determine the Lewis dot structure of the ethyne
Step 1) the first step determines the total number of valence electrons in the carbon atom. the electronic configuration of carbon is as follows,
$\text{ C = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{2}}}\text{ }$
Here, carbon has a 4 valence electron.
Similarly, the electronic configuration of hydrogen is, $\text{ H = 1}{{\text{s}}^{\text{1}}}\text{ }$
Here, carbon has a 1valence electron and requires one electron to attain the nearest noble gas configuration.
Step 2) In this step, draw a skeleton structure of the ethyne molecule. Connect all atoms through the single bond. The central atom will be the atom which has the highest number of valence electrons. Therefore, ethyne carbon is a central carbon atom. The skeleton would involve two carbon atoms surrounded by the two carbon atoms. The skeleton structure of ethyne is,
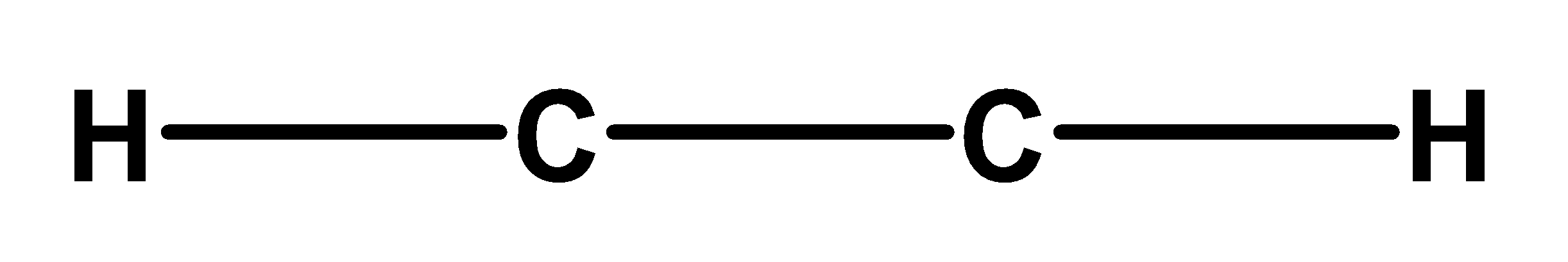
Step 3) In this step we will add all pairs of electrons on the atom. Each carbon atom has 4 valence electrons. Thus total valence electrons from two carbon atoms are $\begin{align}
& \text{ Total valence }{{\text{e}}^{-}}\text{ = 2}\times \text{(V}\text{.E}\text{. of C ) + 2}\times \text{(V}\text{.E}\text{. of H ) } \\
& \Rightarrow \text{ 2}\times \text{ 4 + 2}\times \text{1 = 10 } \\
\end{align}$
Thus we need to place 10 valence electrons on the ethyne molecule. Here six electrons are involved in the bond pairs. We need to place 4 electrons on the skeleton. The hydrogen atom has completed its octet. But each carbon requires 2 more electrons to complete its octet. thus add 4 electrons on the two carbon atoms to complete its octet. It is as shown below,

Ethyne structure can also be represented as,

Note: The Lewis dot structure does not explain the geometry of molecules or how the Bonds are formed, or how the electrons are shared between the atoms. The Lewis dot structure is a simple and limited theory based on the electronic structure and valence numbers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




