
How do you write the equation for the esterification for the esterification reaction for acetic acid and ethyl alcohol forming ethyl acetate?
Answer
533.4k+ views
Hint: The reaction of an Alcohol and a Carboxylic acid to form ester is popularly known as Fischer esterification reaction which generally takes place in presence of acids which catalyze the reaction rate, such an esterification does not generally take place under basic environment due to formation of carboxylate ion which is a highly unreactive species when used as a electrophile.
Complete step by step answer:
In presence of acid the carboxylic acid $ \left( {RCOOH} \right) $ accepts a proton and forms a species which is a really good electrophile thus alcohol $ \left( {ROH} \right) $ now can act as a Nucleophile and attack the Carbonyl $ C $ to form an intermediate species which rapidly forms the final product. The attack on the activated carboxylic acid is the slow step of the reaction and thus is also rate determining in case of esterification reaction. Let us now see a schematic representation of the reaction so as to grasp the way this reaction yields product:
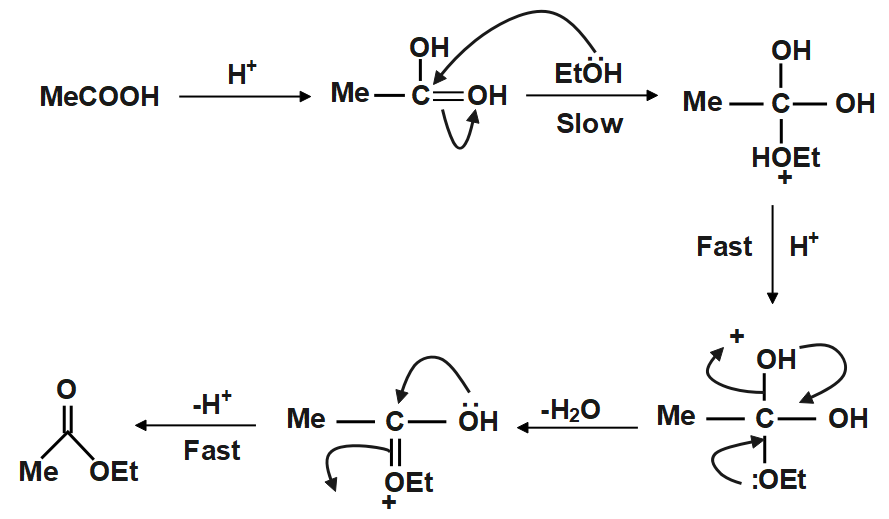
As clearly visible from above the reaction follows a pathway wherein the presence of protons has a massive impact on reaction rate. Here after the formation of an intermediate via a slow step the reaction ups its pace and reaches conclusion at a quicker pace, the above method is one of the most used methods for synthesis of an ester.
Note: We must always be careful on what type of acid we are using because if we use acids that have nucleophilic counter anion associated with them then we will hinder the rate of reaction also we will affect the yield in a negative manner. Another important point to note is the fact that in absence of an acid the above reaction would occur too slowly to give any proper yield.
Complete step by step answer:
In presence of acid the carboxylic acid $ \left( {RCOOH} \right) $ accepts a proton and forms a species which is a really good electrophile thus alcohol $ \left( {ROH} \right) $ now can act as a Nucleophile and attack the Carbonyl $ C $ to form an intermediate species which rapidly forms the final product. The attack on the activated carboxylic acid is the slow step of the reaction and thus is also rate determining in case of esterification reaction. Let us now see a schematic representation of the reaction so as to grasp the way this reaction yields product:

As clearly visible from above the reaction follows a pathway wherein the presence of protons has a massive impact on reaction rate. Here after the formation of an intermediate via a slow step the reaction ups its pace and reaches conclusion at a quicker pace, the above method is one of the most used methods for synthesis of an ester.
Note: We must always be careful on what type of acid we are using because if we use acids that have nucleophilic counter anion associated with them then we will hinder the rate of reaction also we will affect the yield in a negative manner. Another important point to note is the fact that in absence of an acid the above reaction would occur too slowly to give any proper yield.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




