
Write the formula of monomers used in the preparation of dextron.
Answer
530.7k+ views
Hint: Dextron is a polymer that is made up of two monomers and when these two monomers combine there is the elimination of water molecules, so it is a product of condensation polymerization. The monomers used for the preparation of dextron are acids, one acid contains 2 carbon atoms and the other contains 3 carbon atoms.
Complete answer:
We know that polymers are those compounds that have high very high molecular mass and a large number of molecules. These molecules are repeated which are known as monomers. As monomers are the smallest units.
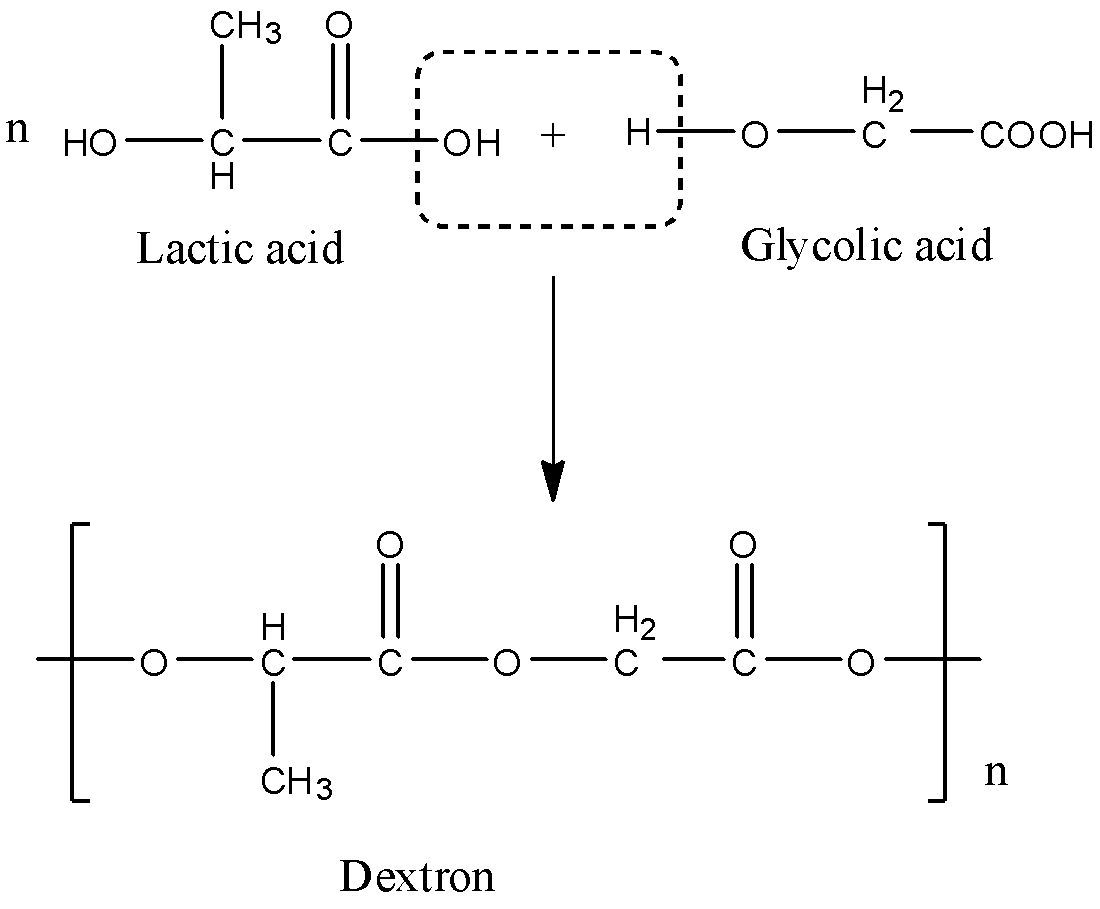
The given polymer is Dextron. Dextron is a polymer that is made up of two monomers and when these two monomers combine there is the elimination of water molecules, so it is a product of condensation polymerization.
The monomers used for the preparation of dextron are acids, one acid contains 2 carbon atoms and the other contains 3 carbon atoms. So, one monomer is Lactic acid whose formula is ${{C}_{3}}{{H}_{6}}{{O}_{3}}$ and the other monomer is Glycolic acid whose formula is ${{C}_{2}}{{H}_{4}}{{O}_{3}}$.
The structural formula of Lactic acid is given below:
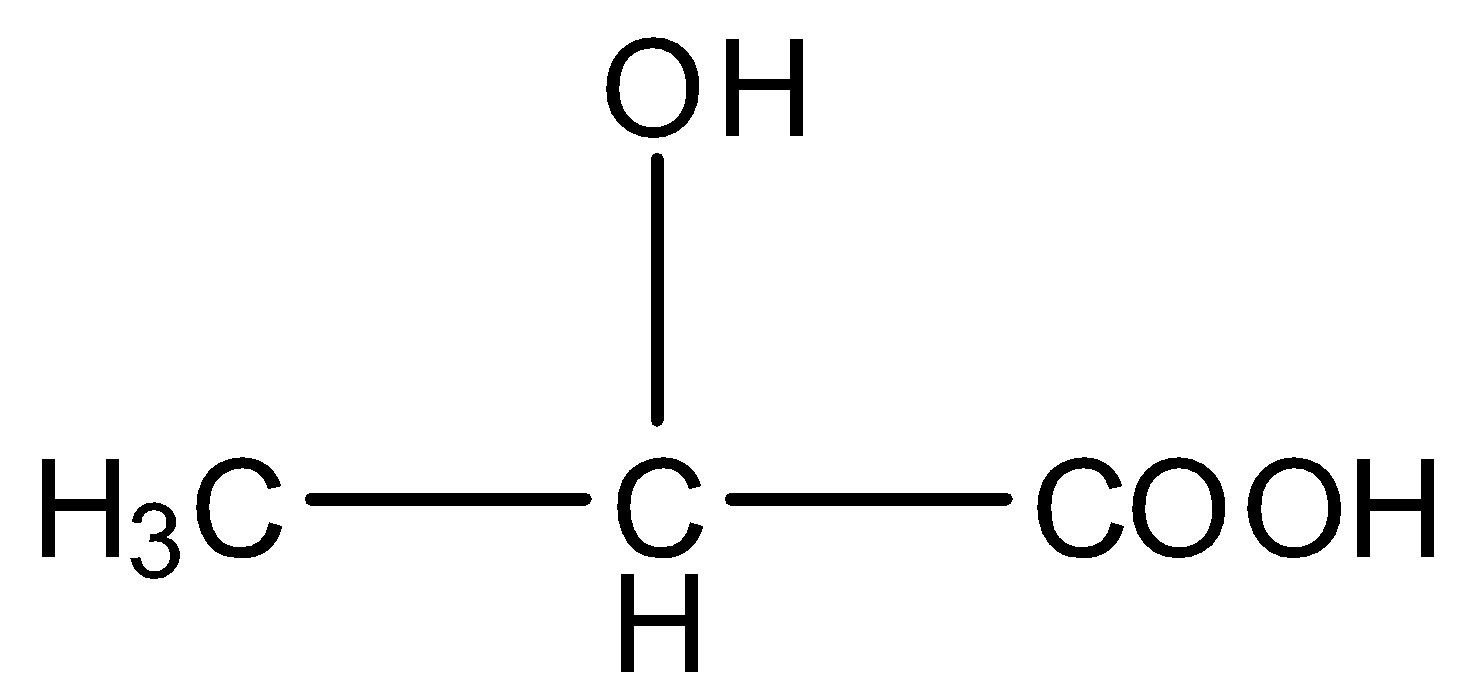
The structural formula of Glycolic acid is given below:
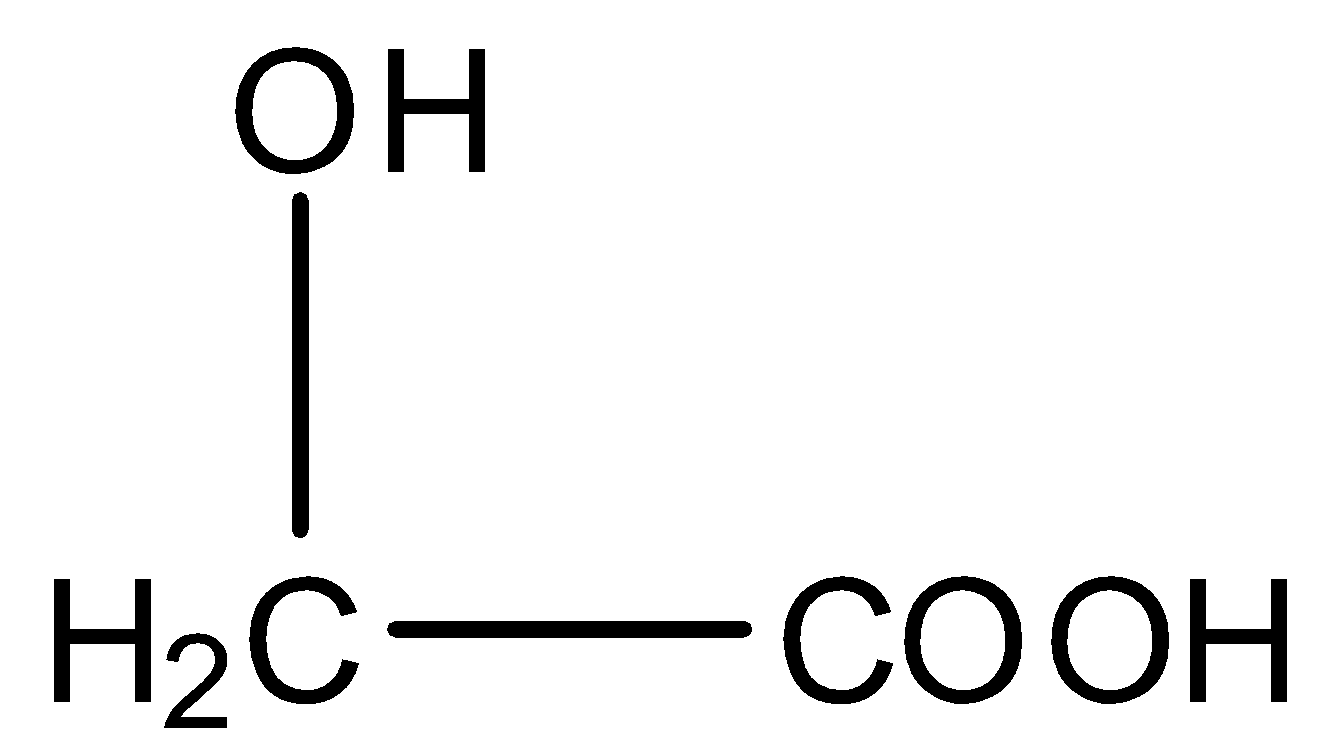
When they combine with each other, the OH group from the acid of one molecule and the H atom from the hydroxyl group of the other molecule will be removed, and this will form a dextron. The reaction is given below:
Note:
Polymers are formed by two processes, i.e., addition polymerization and condensation polymerization, if the polymer is formed without any loss of molecules then it comes under the addition polymerization.
Complete answer:
We know that polymers are those compounds that have high very high molecular mass and a large number of molecules. These molecules are repeated which are known as monomers. As monomers are the smallest units.
The given polymer is Dextron. Dextron is a polymer that is made up of two monomers and when these two monomers combine there is the elimination of water molecules, so it is a product of condensation polymerization.
The monomers used for the preparation of dextron are acids, one acid contains 2 carbon atoms and the other contains 3 carbon atoms. So, one monomer is Lactic acid whose formula is ${{C}_{3}}{{H}_{6}}{{O}_{3}}$ and the other monomer is Glycolic acid whose formula is ${{C}_{2}}{{H}_{4}}{{O}_{3}}$.
The structural formula of Lactic acid is given below:

The structural formula of Glycolic acid is given below:
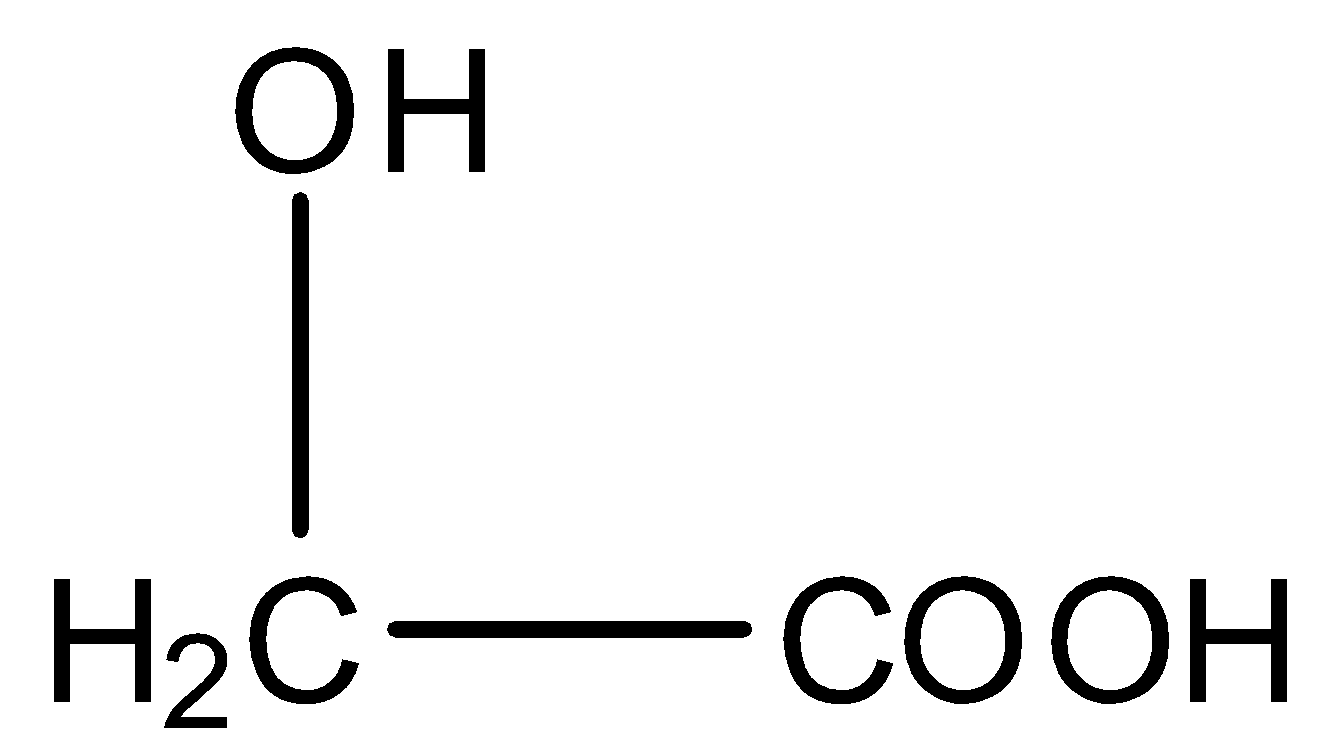
When they combine with each other, the OH group from the acid of one molecule and the H atom from the hydroxyl group of the other molecule will be removed, and this will form a dextron. The reaction is given below:

Note:
Polymers are formed by two processes, i.e., addition polymerization and condensation polymerization, if the polymer is formed without any loss of molecules then it comes under the addition polymerization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




