
Write the IUPAC name: ${(C{H_3})_3}C.C{H_2}.C{H_2}Cl$.
Answer
552.6k+ views
Hint: In the given compound four carbon atoms are present in the parent chain which is represented as butane. The hydrocarbon name is written at the end while naming the IUPAC name. It is an example of a tertiary hydrocarbon compound.
Complete step by step answer:
The IUPAC is the abbreviation used for International union of pure and applied chemistry. In chemical nomenclature, the IUPAC nomenclature is the method used for naming the organic chemical compound.
To determine the name of the chemical compound, given steps are followed.
STEP 1: Numbering of parent chains.
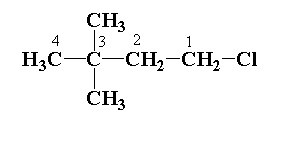
Numbering of chains is done from the highest priority group.
STEP 2: Determining the hydrocarbon chain.
In the given structure, 4 carbon atoms are present in the parent chain. The parent chain is defined as the longest chain which contains the maximum number of carbon atoms. The hydrocarbon chain is butane.
STEP 3: Determine the substituted group.
In the given structure, two methyl groups are attached to the 3rd carbon atom of the parent chain and one chlorine atom is present in the 1st carbon atom.
STEP 4: Determine the IUPAC name of the chemical compound.
The name of the chemical compound is 1-chloro-3, 3-dimethyl butane.
Note:
The number of prefix, suffixes are used to determine the type and position of the functional group in the chemical compound. While numbering the carbon atom, the numbering starts from the highest priority group, usually functional groups. When a functional group like carboxylic acid group –COOH is present, then the carbon of the functional group is included in the parent chain.
Complete step by step answer:
The IUPAC is the abbreviation used for International union of pure and applied chemistry. In chemical nomenclature, the IUPAC nomenclature is the method used for naming the organic chemical compound.
To determine the name of the chemical compound, given steps are followed.
STEP 1: Numbering of parent chains.

Numbering of chains is done from the highest priority group.
STEP 2: Determining the hydrocarbon chain.
In the given structure, 4 carbon atoms are present in the parent chain. The parent chain is defined as the longest chain which contains the maximum number of carbon atoms. The hydrocarbon chain is butane.
STEP 3: Determine the substituted group.
In the given structure, two methyl groups are attached to the 3rd carbon atom of the parent chain and one chlorine atom is present in the 1st carbon atom.
STEP 4: Determine the IUPAC name of the chemical compound.
The name of the chemical compound is 1-chloro-3, 3-dimethyl butane.
Note:
The number of prefix, suffixes are used to determine the type and position of the functional group in the chemical compound. While numbering the carbon atom, the numbering starts from the highest priority group, usually functional groups. When a functional group like carboxylic acid group –COOH is present, then the carbon of the functional group is included in the parent chain.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




