
Write the mechanism of hydration of ethene to yield ethanol.
Answer
522.5k+ views
Hint: We know that in a hydration reaction, the water molecule reacts with a compound which is not saturated but unsaturated to generate an alcohol.
Complete step-by-step solution:
Let us see the example of hydration reaction:
$C_{2}H_{4} + H_{2}SO_{4} \overset{H_{2}O}{\rightarrow} CH_{3}CH_{2}OH$
In this reaction the ethene molecules we see that react with sulphuric acid generating an alcohol, that is ethanol.
In a hydration reaction, the water molecule reacts with an alkene to generate an alcohol. Ethanol can be prepared by the reaction of alkene with the water molecule. Ethene molecules react with sulphuric acid generating an alcohol, that is ethanol.
This conversion of ethene molecule to ethanol molecule involves three steps. The three steps are:
1) Electrophilic attack on the hydronium ion takes place which pronates the ethene to form a carbocation.
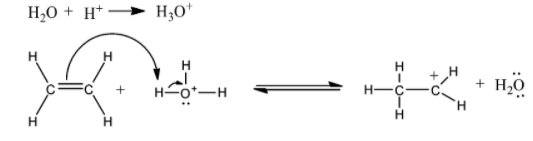
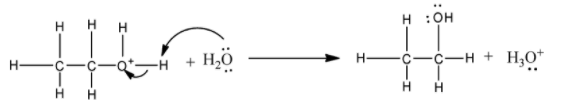
2) In the second step, the carbocation is attacked by the water molecule.

3) The deprotonation lastly generates the ethanol.
Note: Students may get confused between hydration reaction and hydrolysis reaction. In a hydration reaction, the water molecule reacts with an unsaturated compound to generate an alcohol but in hydrolysis reaction chemical bonds are broken due to addition of water.
Complete step-by-step solution:
Let us see the example of hydration reaction:
$C_{2}H_{4} + H_{2}SO_{4} \overset{H_{2}O}{\rightarrow} CH_{3}CH_{2}OH$
In this reaction the ethene molecules we see that react with sulphuric acid generating an alcohol, that is ethanol.
In a hydration reaction, the water molecule reacts with an alkene to generate an alcohol. Ethanol can be prepared by the reaction of alkene with the water molecule. Ethene molecules react with sulphuric acid generating an alcohol, that is ethanol.
This conversion of ethene molecule to ethanol molecule involves three steps. The three steps are:
1) Electrophilic attack on the hydronium ion takes place which pronates the ethene to form a carbocation.

2) In the second step, the carbocation is attacked by the water molecule.

3) The deprotonation lastly generates the ethanol.

Note: Students may get confused between hydration reaction and hydrolysis reaction. In a hydration reaction, the water molecule reacts with an unsaturated compound to generate an alcohol but in hydrolysis reaction chemical bonds are broken due to addition of water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




