
Write the molecular and structural formula of BHA and BHT.
Answer
587.1k+ views
Hint:
Antioxidants are naturally occurring chemicals in food that help to counter the detrimental effects of oxygen free radicals, which form during normal metabolism and through external factors. BHA and BHT are examples of synthetic antioxidants. It comes under the phenol family.
Complete step by step answer:
Butylated hydroxyanisole is abbreviated as BHA while Butylated hydroxytoluene is abbreviated as BHT.
Molecular formula of BHA is \[{{\text{C}}_{11}}{{\text{H}}_{16}}{{\text{O}}_2}\]while molecular formula of BHT is ${{\text{C}}_{15}}{{\text{H}}_{24}}{\text{O}}$. The structural formula of BHA and BHT is given below:
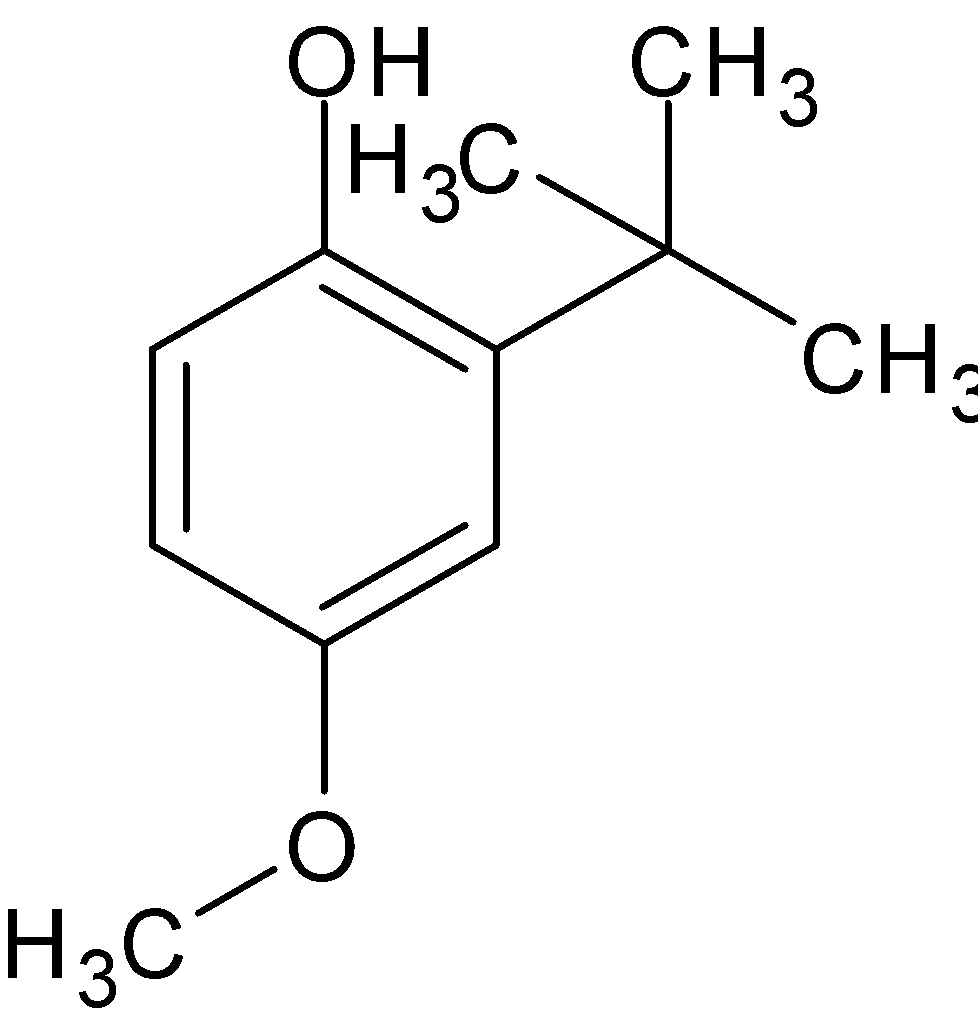
Butylated hydroxyanisole (BHA)
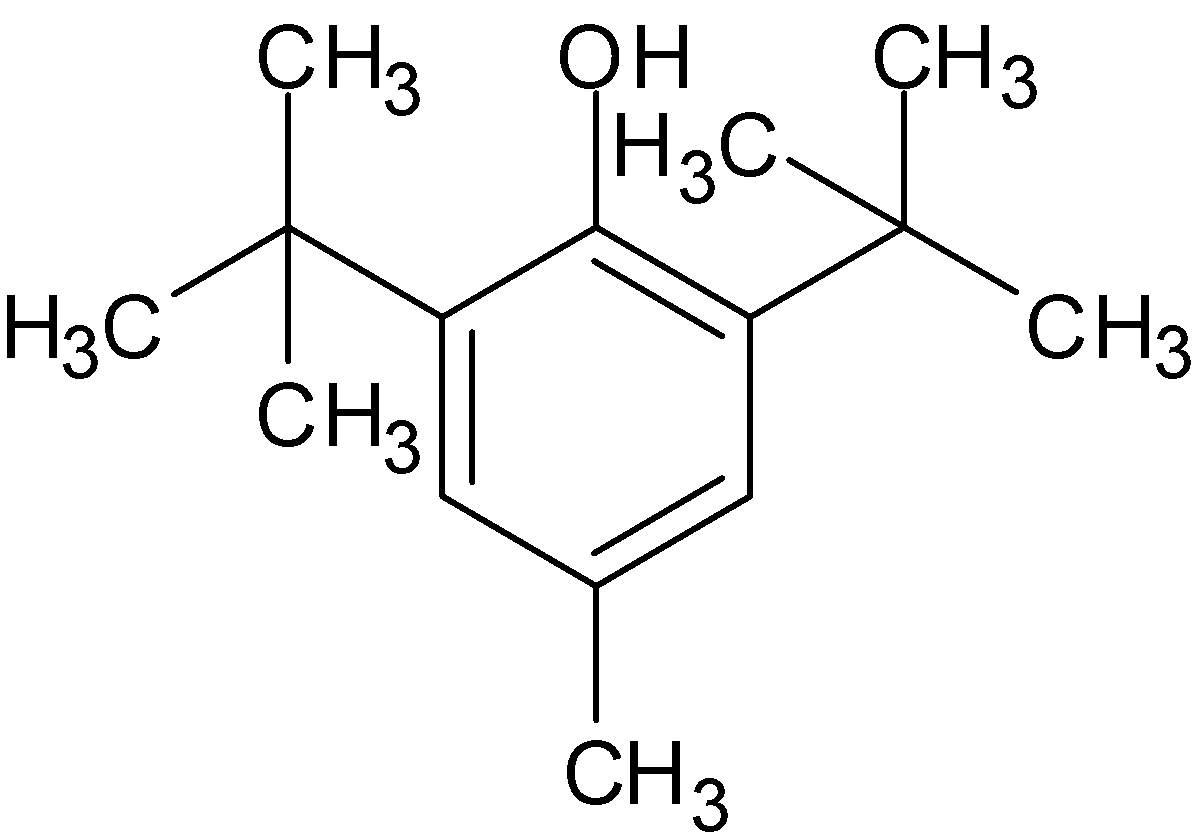
Butylated hydroxytoluene
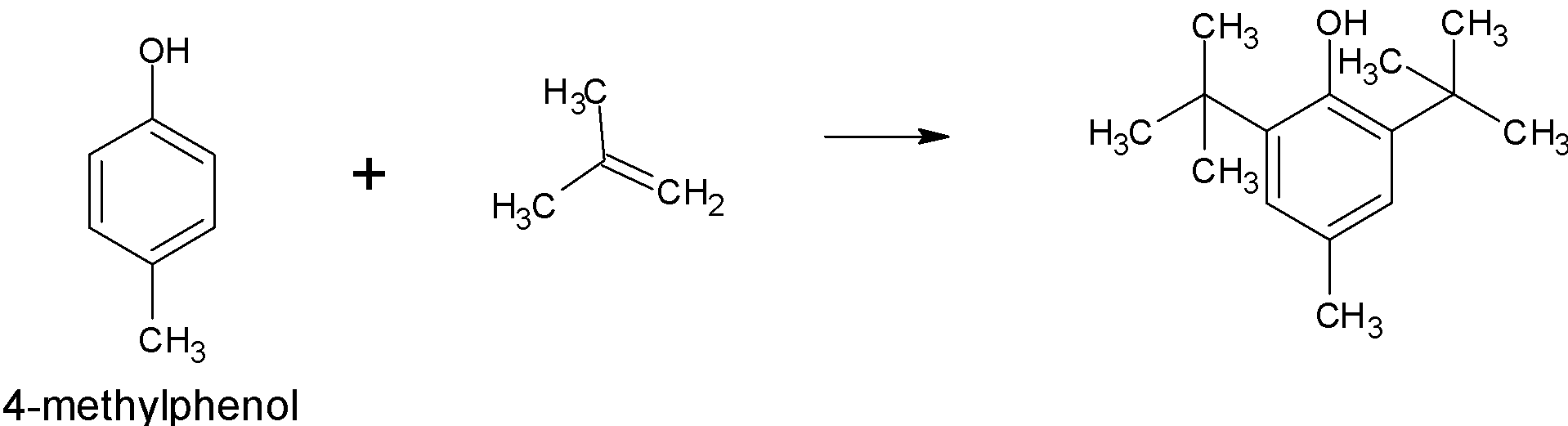
BHT is prepared by following reaction:
BHA is fat soluble, water soluble, white solid and volatile. It is frequently used for preservation of coconut. It is good in baking because of its stability to heat and its mild alkaline conditions. It is useful in protecting the odor and flavor of essential oils.
BHT is a white crystalline solid. It is also fat soluble. It is usually combined with BHA to provide greater antioxidant property. BHT when used at high temperature for a long period of time, may impart undesirable odors. It possesses antimicrobial activity.
Note:
Free radical formation is controlled by various compounds called antioxidants. The ability of antioxidants is limited that this damage can become cumulative and debilitating. They are capable of stabilizing or deactivating free radical before they attack cells. BHA and BHT react with oxygen free radicals and form non-radical products, since these free radicals are very reactive and cause damage to cells.
Antioxidants are naturally occurring chemicals in food that help to counter the detrimental effects of oxygen free radicals, which form during normal metabolism and through external factors. BHA and BHT are examples of synthetic antioxidants. It comes under the phenol family.
Complete step by step answer:
Butylated hydroxyanisole is abbreviated as BHA while Butylated hydroxytoluene is abbreviated as BHT.
Molecular formula of BHA is \[{{\text{C}}_{11}}{{\text{H}}_{16}}{{\text{O}}_2}\]while molecular formula of BHT is ${{\text{C}}_{15}}{{\text{H}}_{24}}{\text{O}}$. The structural formula of BHA and BHT is given below:

Butylated hydroxyanisole (BHA)

Butylated hydroxytoluene
BHT is prepared by following reaction:

BHA is fat soluble, water soluble, white solid and volatile. It is frequently used for preservation of coconut. It is good in baking because of its stability to heat and its mild alkaline conditions. It is useful in protecting the odor and flavor of essential oils.
BHT is a white crystalline solid. It is also fat soluble. It is usually combined with BHA to provide greater antioxidant property. BHT when used at high temperature for a long period of time, may impart undesirable odors. It possesses antimicrobial activity.
Note:
Free radical formation is controlled by various compounds called antioxidants. The ability of antioxidants is limited that this damage can become cumulative and debilitating. They are capable of stabilizing or deactivating free radical before they attack cells. BHA and BHT react with oxygen free radicals and form non-radical products, since these free radicals are very reactive and cause damage to cells.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




