
Write the monomers of the following polymers:
(a) Bakelite
(b) Nylon - 6,6
Answer
570k+ views
Hint: Draw the expanded structure of the polymers, Bakelite and Nylon - 6,6. Now determine the repeating compound or compounds. With this you can determine the monomer compounds. Now name them in accordance with the IUPAC nomenclature.
Complete Solution :
Polymerization is a process of reacting monomer molecules together in a chemical reaction to form long polymer chains .
- In chemical compounds, polymerization can occur by various types of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and the steric effects that are present.
- In simple polymerizations, alkenes form polymers through relatively simple free radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize and the steric hindrance offered by neighboring groups.
The expanded structures of Bakelite and Nylon-6,6 respectively are given below:
Bakelite:
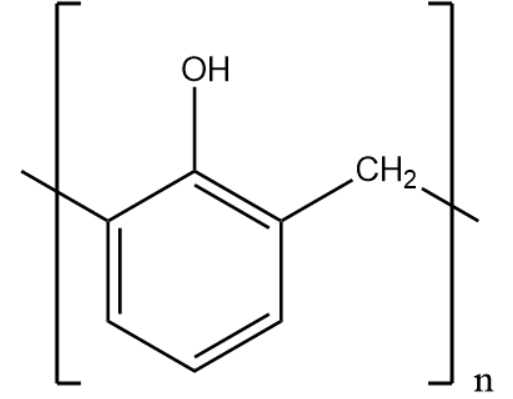
Nylon -6,6:
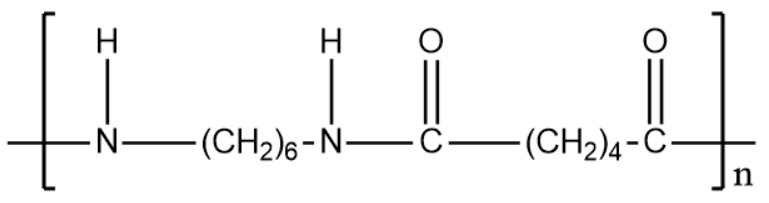
Based on the expanded structure we can identify the monomers.
Both the polymer consists of 2 monomers and thus are examples of copolymerization.
The monomers of Bakelite are phenol and formaldehyde.
The monomers of Nylon -6,6 are hexamethylenediamine and adipic acid.
Additional Information.: The two main classes of polymerisation reaction mechanisms are step-growth and chain growth polymerisation.
In step-growth polymerisation, the pairs of reactants combine at each step to form a longer polymer chain. The average molar mass increases slowly, long chains are formed only at the end of the reaction.
However, in case of chain growth polymerisation the addition of monomer to the growing chain with active centre (like free radical, cation, anion) is the chain extension step. Long chains are formed from the starting of the reaction only.
Note: The polymer name Nylon - 6,6 denotes that two monomers having 6 carbon atoms each are used to form this particular polymer. With this we can identify the monomers of Nylon -6,6.
Complete Solution :
Polymerization is a process of reacting monomer molecules together in a chemical reaction to form long polymer chains .
- In chemical compounds, polymerization can occur by various types of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and the steric effects that are present.
- In simple polymerizations, alkenes form polymers through relatively simple free radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize and the steric hindrance offered by neighboring groups.
The expanded structures of Bakelite and Nylon-6,6 respectively are given below:
Bakelite:

Nylon -6,6:

Based on the expanded structure we can identify the monomers.
Both the polymer consists of 2 monomers and thus are examples of copolymerization.
The monomers of Bakelite are phenol and formaldehyde.
The monomers of Nylon -6,6 are hexamethylenediamine and adipic acid.
Additional Information.: The two main classes of polymerisation reaction mechanisms are step-growth and chain growth polymerisation.
In step-growth polymerisation, the pairs of reactants combine at each step to form a longer polymer chain. The average molar mass increases slowly, long chains are formed only at the end of the reaction.
However, in case of chain growth polymerisation the addition of monomer to the growing chain with active centre (like free radical, cation, anion) is the chain extension step. Long chains are formed from the starting of the reaction only.
Note: The polymer name Nylon - 6,6 denotes that two monomers having 6 carbon atoms each are used to form this particular polymer. With this we can identify the monomers of Nylon -6,6.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




