
Write the names and structures of the monomers of the following polymers:
(A) Buna-S
(B) Buna-N
(C) Dacron
(D) Neoprene
Answer
592.5k+ views
Hint: The polymers are very large molecules which are made up of many repeating molecular units called monomers. Buna-S, Dacron, Buna-N are condensation polymers and Neoprene is an Addition polymer.
Complete step by step answer:
1. Buna-S:
Buna-S is called butadiene-styrene copolymer. The monomers of this are: 1,3-Butadiene and Styrene.
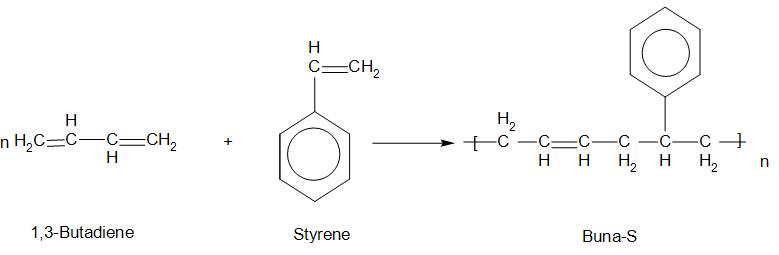
- There are several properties of Buna-S like:
- it is having high abrasion resistance, high resilience and high bearing capacity.
- It gets oxidised in the presence of traces of ozone. And it is found that it swells in oils and solvents.
- It is used in the manufacture of car tyres, wire and cable, floor tiles etc.
2. Buna-N:
Buna-N is known as nitrile rubber. The monomers of this are: acrylonitrile and 1,3butadiene
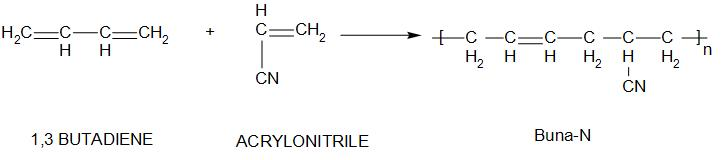
- There are several properties of Buna-N like:
- It shows excellent resistance to heat, sunlight, salts, acid and oil.
-The copolymer is low in tensile strength; its tensile strength can be increased by adding reinforcing pigment.
-It is less elastic, rubbery than that of the natural rubber.
3. Dacron:
Dacron is called Polyethylene terephthalate. The monomers of this are: ethylene glycol and terephthalic acid
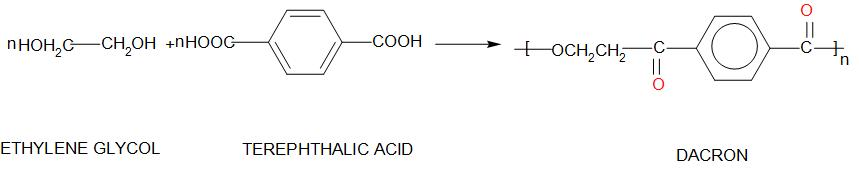
-There are several properties of Dacron like
- it is strong and has good elasticity
- it is insoluble in almost all inorganic as well as organic solvents.
- it is found to be resistant to many of the chemical, microorganisms.
4. Neoprene:
It is also called polychloroprene. The monomers of this are: Chloroprene and 2-chloro-1,3-butadiene.
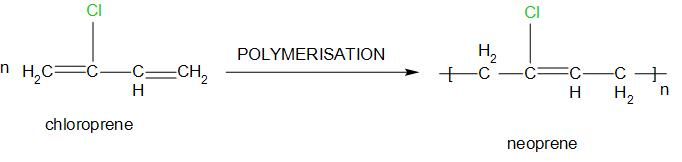
- It performs well in contact with many chemicals and oil.
- It is having strong physical toughness.
Note:
- Always keep in mind that in case of addition polymers the same monomer repeats itself and “adds up” while in case of condensation polymers, two different monomers join together to form the polymer.
Complete step by step answer:
1. Buna-S:
Buna-S is called butadiene-styrene copolymer. The monomers of this are: 1,3-Butadiene and Styrene.

- There are several properties of Buna-S like:
- it is having high abrasion resistance, high resilience and high bearing capacity.
- It gets oxidised in the presence of traces of ozone. And it is found that it swells in oils and solvents.
- It is used in the manufacture of car tyres, wire and cable, floor tiles etc.
2. Buna-N:
Buna-N is known as nitrile rubber. The monomers of this are: acrylonitrile and 1,3butadiene

- There are several properties of Buna-N like:
- It shows excellent resistance to heat, sunlight, salts, acid and oil.
-The copolymer is low in tensile strength; its tensile strength can be increased by adding reinforcing pigment.
-It is less elastic, rubbery than that of the natural rubber.
3. Dacron:
Dacron is called Polyethylene terephthalate. The monomers of this are: ethylene glycol and terephthalic acid

-There are several properties of Dacron like
- it is strong and has good elasticity
- it is insoluble in almost all inorganic as well as organic solvents.
- it is found to be resistant to many of the chemical, microorganisms.
4. Neoprene:
It is also called polychloroprene. The monomers of this are: Chloroprene and 2-chloro-1,3-butadiene.

- It performs well in contact with many chemicals and oil.
- It is having strong physical toughness.
Note:
- Always keep in mind that in case of addition polymers the same monomer repeats itself and “adds up” while in case of condensation polymers, two different monomers join together to form the polymer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




