
Write the number of hydroxyl groups present in $\alpha -D-(+)-Glucopyranose$ (Trans).
Answer
582.9k+ views
Hint: Glucopyranose is a carbohydrate it is the pyranose form of glucose. When D-glucose is crystallized from methanol $\alpha -D-(+)-Glucopyranose$ is formed.
Complete step by step answer:
Glucopyranose is the pyranose form of glucose. Sugars in chemistry is described using the formula given by Haworth. The heterocyclic ring of pyran and furan are resembled by the pyranose and the furanose oxide ring respectively, in which one of the positions is occupied by oxide.
Pyronose is use in the prediction of structure of saccharides which are six membered rings. These six member rings have five carbon and one oxygen atom. There can be other extra carbons attached to the ring as substituents.
To draw the structure of $\alpha -D-(+)-Glucopyranose$ a pyranose ring having carbon and one oxygen atom is drawn first. These structures were suggested by Haworth in Haworth formula.
In the pyranose ring at the terminal position $C{{H}_{2}}OH$ group is added. Groups present on the left hand side in the Fischer projection are placed above the plane of the ring and the groups present at the left hand side are placed below the plane of the ring. Now the position of the OH group differs at the ${{C}_{1}}$ carbon, OH group down the plane of the ring forms the $\alpha -D-(+)-Glucopyranose$.
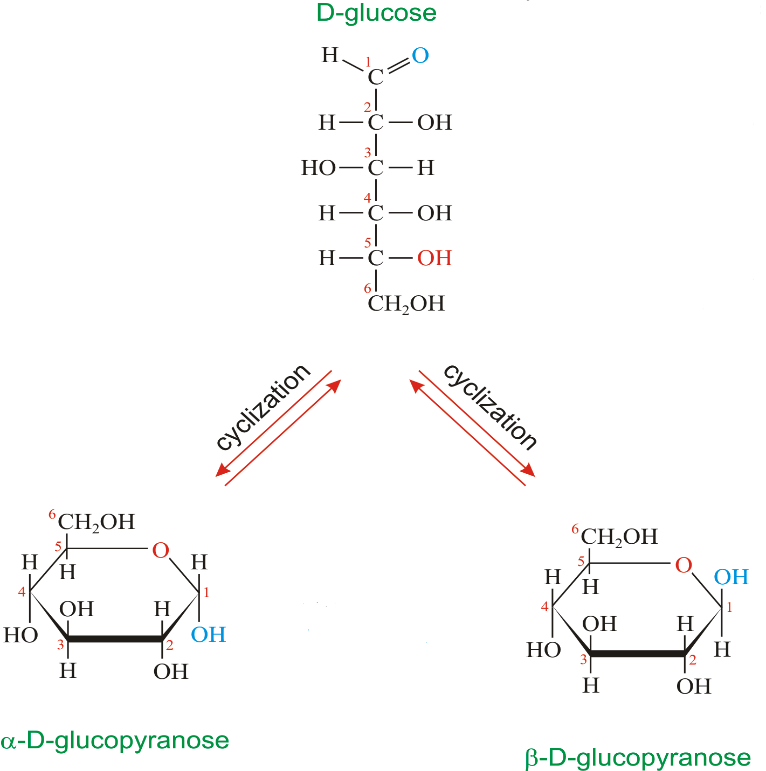
From the structure we can say that the number of hydroxyl groups present in $\alpha -D-(+)-Glucopyranose$ is 5.
Note: The structure of $\beta -D-(+)-Glucopyranose$ can also be drawn using the method mentioned above the only difference is that OH group at the ${{C}_{1}}$ carbon will be placed above the plane of the ring.
Complete step by step answer:
Glucopyranose is the pyranose form of glucose. Sugars in chemistry is described using the formula given by Haworth. The heterocyclic ring of pyran and furan are resembled by the pyranose and the furanose oxide ring respectively, in which one of the positions is occupied by oxide.
Pyronose is use in the prediction of structure of saccharides which are six membered rings. These six member rings have five carbon and one oxygen atom. There can be other extra carbons attached to the ring as substituents.
To draw the structure of $\alpha -D-(+)-Glucopyranose$ a pyranose ring having carbon and one oxygen atom is drawn first. These structures were suggested by Haworth in Haworth formula.
In the pyranose ring at the terminal position $C{{H}_{2}}OH$ group is added. Groups present on the left hand side in the Fischer projection are placed above the plane of the ring and the groups present at the left hand side are placed below the plane of the ring. Now the position of the OH group differs at the ${{C}_{1}}$ carbon, OH group down the plane of the ring forms the $\alpha -D-(+)-Glucopyranose$.

From the structure we can say that the number of hydroxyl groups present in $\alpha -D-(+)-Glucopyranose$ is 5.
Note: The structure of $\beta -D-(+)-Glucopyranose$ can also be drawn using the method mentioned above the only difference is that OH group at the ${{C}_{1}}$ carbon will be placed above the plane of the ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




